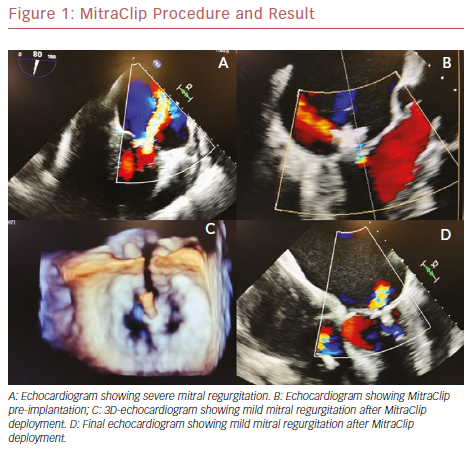
The field of mitral valve disease diagnosis and management is in constant change. New understanding of disease pathology and progression, with improvements in and increased use of sophisticated imaging modalities have led to more complex treatments. Transcatheter mitral valve repair with a MitraClip device is resulting in good outcomes in patients with primary mitral regurgitation who are at high surgical risk.1
In primary mitral regurgitation, surgical repair of the mitral valve and its apparatus is the standard of care. However, surgical treatment of secondary mitral regurgitation has not been demonstrated to be better than medical therapy regarding improvement in quality of life or survival, and mitral valve surgery treatment has a weak class IIb recommendation according to 2017 European Society of Cardiology and American Heart Association/American College of Cardiology (ESC/ACC/AHA) guidelines for the management of patients with valvular heart disease.2,3 In this paper, we review recently published articles on MitraClip therapy.
Pathophysiology
Mitral regurgitation (MR) is classified as either primary or secondary. Primary and secondary MR are two different disease states.3 Primary MR is the result of pathology of one or more components of the mitral valve apparatus. In patients with secondary MR, the chordae tendineae and mitral valve leaflets are structurally normal, and mitral regurgitation results from dilatation or remodeling of the left ventricle, causing either leaflet tethering and/or impaired coaptation. The main cause of the disease is the underlying cardiomyopathy, and the regurgitation is probably a signal or marker of the disease; the ventricle, not the valve, is the culprit. The presence of chronic secondary MR is associated with an impaired prognosis.4–6 Secondary MR is strongly associated with hospitalization for heart failure (HF) and mortality despite treatment with medical therapy alone.7,8 No data have yet demonstrated whether a lack of improvement in left ventricular function affects survival.9,10
In patients with secondary MR, which is mainly a disease of the left ventricle, treatment options have advanced significantly. The use of transcatheter techniques for both repair and replacement is expected to expand substantially in the next few years.1
MitraClip Procedure
The percutaneous mitral valve repair procedure involves of the implantation of a dedicated device – the MitraClip – in both mitral cuspids at the same time; attachment of the leaflets helps to reduce regurgitant flow. It is performed under general anesthesia, under the guidance of transesophageal echocardiography (TEE) and fluoroscopy. A trans-septal puncture procedure is performed to gain access to the left atrium. The mitral leaflets are grasped onto the MitraClip and the device is closed, resulting in a fixed approximation of the mitral leaflets. Adequate reduction of mitral regurgitation to a grade of 2+ or less is considered successful according to intraoperative TEE. If the reduction of the degree of mitral regurgitation is still inadequate, a second device may be deployed.11–13 Figure 1 shows the MitraClip.
Clinical Studies
Prospective studies suggest that percutaneous mitral valve repair with a MitraClip may decrease symptoms and improve functional capacity and quality of life in patients with secondary MR.14,15 However, these studies were not randomized controlled trials. Therefore, current guidelines do not strongly recommend percutaneous repair for secondary MR.2,3
The Endovascular Valve Edge-to-Edge Repair Study (EVEREST II) trial randomized 279 relatively low-risk patients (left ventricle ejection fraction >60%, and most with New York Heart Association [NYHA] class II or III symptoms) to a MitraClip device group (184 patients, 73% primary MR, 27% secondary MR) or a mitral valve surgery group (95 patients, 73% primary MR, 27% secondary MR).16 Although the degree of mitral regurgitation reduction was lower with the MitraClip procedure than surgical mitral valve repair, reduction of mitral regurgitation to ≤2+ was observed in most of the MitraClip patients, an effect that was sustained over 5 years.
The MitraClip group had similar 1- and 5-year effectiveness as the mitral valve surgery group in the subset of patients with secondary MR, but not in those with primary MR. Patients treated with a MitraClip more commonly required surgery for residual mitral regurgitation during the first year after treatment but, between 1 and 5 years of follow-up, both groups experienced low rates of surgery.17
In the Getting Reduction of Mitral Insufficiency by Percutaneous Clip Implantation (GRASP) registry, the safety and efficacy of the MitraClip technique were demonstrated in degenerative and secondary MR (3+–4+) in both men and women who could not have repair surgery or were at a high surgical risk.18,19 Its efficiency has also been demonstrated in patients aged over 75 years.20 However, the GRASP Registry was not a randomized controlled clinical study, and there was no control group.
In the ACCESS-Europe Two-Phase Observational Study of the MitraClip System in Europe (ACCESS EU) trial, a total of 567 patients with significant mitral valve regurgitation underwent the MitraClip procedure. Patients had NYHA functional class III or IV symptoms. Left ventricular ejection fraction <40% was present in 52.7% of patients, and 5% of patients had an ejection fraction of <20%. The MitraClip device implant rate was 99.6%. There was a reduction in the severity of MR at 12 months, compared with baseline (p< 0.0001), with 78.9% of patients free from MR, and severity of >2+ at 12 months. At 12 months, 71.4% of patients had either NYHA functional class II or class I symptoms. Although the patients undergoing MitraClip therapy were elderly and were at high surgical risk, the MitraClip procedure was demonstrated to be effective, with low rates of adverse events and hospital mortality.21
Recently, two large studies with similar characteristics but with conflicting results were published, and comparing them helps us to better understand what kind of patients should be treated with the transcatheter mitral technique.
MITRA-FR Trial
In the MitraClip Device in Patients With Severe Secondary Mitral Regurgitation (MITRA-FR) trial, a multicenter, randomized controlled study, patients presenting with secondary MR were randomized to either a MitraClip group (152 patients, MitraClip plus medical therapy) or a control group (152 patients, medical therapy alone).10,22 Patients had to have a left ventricular ejection fraction of 15–40% and chronic HF symptoms (NYHA functional class II, III, or IV).
In the MitraClip group, implantation was attempted in 144 patients, and technical success in fitting the device was achieved in 138 of these patients (95.8%). At the time of discharge, the patients were evaluated with regard to the severity of MR in the intervention group. Of these patients, 113 (91.9%) had a reduction in MR to 2+ or lower, and 93 patients (75.6%) had a substantial reduction to 1+ or 0.
The composite primary outcome of death from any cause or unplanned hospitalization for HF at 12 months occurred in 83 patients (54.6%) in the MitraClip group, and in 78 patients (51.3%) in the control group (p=0.53). At 12 months, there had been 37 deaths (24.3%) in the MitraClip group and 34 (22.4%) in the control group. In the MitraClip group, 74 patients (48.7%) had an unplanned hospitalization for HF, compared with 72 patients (47.4%) in the control group (p=ns).10,22 The MITRA-FR trial investigators concluded that percutaneous mitral valve repair plus medical therapy offered no advantage over medical therapy alone in patients with HF with a reduced ejection fraction and secondary mitral valve regurgitation.
COAPT Trial
Another large study, the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy (COAPT) trial, evaluated the reduction in risk of HF hospitalization and mortality.11,23 It included 614 patients with HF and moderate to severe or severe functional MR who were randomized to percutaneous mitral valve repair (MitraClip group: n=302; mean age 72 years; 67% men) or medical therapy alone (the control group: n=312; mean age 73 years; 62% men). The primary effectiveness endpoint was all hospitalizations for HF within 24 months of follow-up. Patients had non-ischemic or ischemic cardiomyopathy with a left ventricular ejection fraction of 20–50%, moderate to severe (grade 3) or severe (grade 4) secondary MR confirmed with echocardiography, and were symptomatic (NYHA functional class II, III, or IV) despite the use of guideline-directed medical therapy at maximally tolerated doses.
MitraClip implantation was attempted in 293 of the 302 patients (97.0%) in the device group, with one or more clips implanted in 287 patients. The rate of freedom from device-related complications at 12 months was 96.6%.23 The rate of HF hospitalizations per year was lower in the device group than the control group (35.8% versus 67.9%, p<0.001). In addition, the secondary endpoint of 2–year mortality was significantly lower among MitraClip patients at 29.1% versus 46.1% for the controls. The absolute risk reduction in all-cause mortality in patients receiving the MitraClip in the COAPT trial was 17%, which translates to a number needed to treat of six to prevent one death over 2 years. It was the first therapy shown to improve the prognosis in high-risk patients with secondary MR due to underlying left ventricular dysfunction.
Comparison of MITRA-FR and COAPT Trials
MitraClip had been demonstrated as a successful therapy before in patients who were at high surgical risk. The difference in the results of these two randomized studies are likely to be related to patient selection, number of patients, medication changes during the study, duration of follow-up and operator experience. Rigorously screening patients in a non-blinded trial undoubtedly increases the chances of selection bias but may not explain the dramatic effect sizes noted in the COAPT trial.9,24 The two studies are complementary to one another rather than controversial.
Patient Selection and Follow-up
MITRA-FR was the first prospective randomized trial of functional MR catheter-based treatment, and it concluded that percutaneous mitral valve repair was of no benefit. The MitraClip procedure was deemed safe and efficient and it decreased regurgitation but did not improve clinical outcomes according to this study. However, a lot of data on patients’ follow-up echocardiographic results were missing.10 Conversely, in the COAPT trial, the clinical, functional, echocardiographic and health status outcomes were all congruent.23
Safety
The MitraClip is a less invasive and a much safer therapy than surgery in this group of patients. An important result of the study was it showed how safe the procedure is. The complication rate was only about 3% in a population with a high mortality risk. Device failure or complication rates were lower than expected.10,18,19,23
The low mortality risk associated with the MitraClip procedure was demonstrated in a recently published meta-analysis that identified 21 studies in high-risk patients, representing real-world experience, where the MitraClip was used in 3,198 patients and mitral surgery in 53,265, with a mean age of 74 years. Procedural success was observed in 96% of patients who underwent MitraClip implantation and 98% in the surgical group.25 The MitraClip procedure is safe, even in a critically ill patient group.13
Hemodynamics
The severity of mitral regurgitation accepted in COAPT was substantially greater and the ventricles were not severely dilated in this study. Comparing the control arms of COAPT and MITRA-FR trials at 1-year time points reveal that rates of all-cause mortality were similar (23% and 22% respectively), which suggests the patient population enrolled in the two RCTs were not drastically different.
The degree of mitral regurgitation among patients selected in COAPT was more severe than in MITRA-FR. Mean effective regurgitant orifice area was 41 mm2 in COAPT and 31 mm2 in MITRA-FR. The mean ejection fraction in the MitraClip implantation arms was similar (31.3% in COAPT and 33.3% in MITRA-FR). Notably, the indexed left-ventricular end-diastolic volume was higher in MITRA-FR (135 ± 37 ml/m2) than in COAPT (10 ± 34 ml/m2).
These are key differences between the trials as far as identifying which patients would benefit the most. However, both these indexed left ventricular volumes would be considered to indicate severely dilated ventricles, as per the echocardiographic guidelines.2 More importantly, subgroup analyses in COAPT in patients with left ventricular volumes similar to MITRA-FR showed similar reductions, so may not explain the discordant results.
Medical Treatment and pro-BNP
HF medications were allowed to be changed in the MITRA-FR trial but, in COAPT, patients were on maximally tolerated guideline-directed medical therapy at baseline with few major changes during follow-up. Also N-terminal pro-B-type natriuretic peptide (pro-BNP) levels (a well-accepted surrogate marker of left ventricular stress) were higher at baseline in the COAPT trial population.9
Volume of Cases and Center Experience
Operator experience is important as it affects outcomes. Centers with a higher number of patients treated with MitraClip have the best results; the GRASP registry demonstrated that high-volume centers had more successful results.18,20 The procedure is invasive and difficult, and requires a steep learning curve.
According to the MITRA-FR trial investigators, centers were required to be experienced in the MitraClip procedure and to have performed it at least five times before they could be selected as a trial site.21 This may be a limitation of MITRA-FR results. Five cases may not be enough to guarantee adequate experience in carrying out the MitraClip procedure.
Validation Trials and Guidelines Recommendation
Following the MITRA-FR and COAPT studies, ESC/ACC/AHA guidelines’ recommendations may change, but the MitraClip will be used in more patients regardless. The results are pending for an ongoing 420-patient study, Clinical Evaluation of the Safety and Effectiveness of the MitraClip System in the Treatment of Clinically Significant Functional Mitral Regurgitation (RESHAPE-HF2; NCT02444338), which randomized patients in a similar way as the COAPT trial and has run since 2015 in Europe.26 If the findings of this study are similar to those of the COAPT trial, it would open doors to performing the MitraClip procedure in appropriately selected patients.
Certainly, the COAPT trial results should not be extrapolated to a broader secondary patient population with MR and HF. In a shared decision-making model, only carefully selected patients, using a heart-team approach, should undergo the MitraClip procedure for treatment of secondary MR.
Whether the conflicting results of the MITRA-FR and COAPT trials are down to a case of intervening early or late in the course of the disease remains to be established. Until then, patients with MR secondary to HF who meet the COAPT trial criteria may benefit from a MitraClip. HF specialists need to identify these patients and refer them to the heart team.
In the past, this patient population was mostly treated with medical therapy. Now, the MitraClip may become standard care for these patients. It can substantially improve their exercise capacity and quality of life, reduce their need for HF hospitalization, and improve their survival. HF is the first overall cause of morbidity and mortality in developed countries, and has tremendous cost implications for resource utilization.
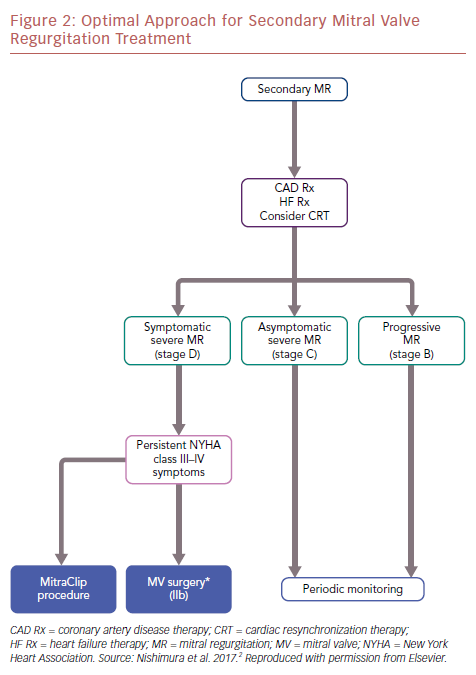
The differences between MITRA-FR and COAPT also allow us to understand this complex disease better. The optimal approach for secondary MR treatment is shown in an algorithm (Figure 2).
Conclusion
MitraClip has been demonstrated to be a successful therapy for patients at high surgical risk. The MitraClip procedure can be safely and effectively performed in patients with severe secondary MR. Two recent large randomized studies (the MITRA-FR and COAPT trials) have conflicting results, but they should be interpreted as complementary trials. Although the MITRA-FR trial did not show significant differences between the intervention and control groups, the COAPT trial demonstrated a reduction in hospitalization and mortality rates in patients with HF and secondary MR in the MitraClip group compared with those receiving medical therapy alone. Cardiologists should individualize treatments in accordance with patient characteristics, and select patients who would benefit from the procedure accurately.