Ionizing radiation is a workplace hazard that goes undetected. In the cardiac catheterization laboratory, where various interventional and electrophysiological procedures are done, physicians and other staff are exposed to ionizing radiation daily. The amount of radiation exposure in the catheterization laboratory is exponentially more compared to other departments using X-ray and fluoroscopy, which increases even more in complex cases. Staff are at a higher lifetime risk of various medical conditions, ranging from cataracts to malignancies. The two major sources of radiation exposure to staff in the cardiac catheterization laboratory are scattered X-ray photons and X-ray tube leakage.
As per the original definitions given by International Commission on Radiological Protection, radiation effects were classified into stochastic (random) and non-stochastic (or deterministic). Currently, the classification has been changed to stochastic and tissue reactions, but there is an overlap of common reactions, such as thyroid cancer and cataracts, into both types.1
Radiation in the catheterization laboratory is generated using two different modes: fluoroscopy or cine angiography (cine). Fluoroscopy is used for catheter placement and involves 95% of the total X-ray operation time, but only causes 40% of the total radiation exposure to staff and patients.2 Cine is used to acquire diagnostic images and to generate a permanent record of the procedure, and although representing only 5% of the total X-ray tube operation time, 60% of the total radiation exposure to staff and patients occur during cine. This is primarily due to the use of a relatively high dose required to record onto film.
It has been revealed that there may be an excess risk of brain tumors among interventional cardiologists.3,4 The lens of the eye is a region of particular interest, with several studies showing an increased incidence of cataracts among catheterization laboratory staff.5–7 A recent study of radiation-induced cataracts documented that 52% of interventional cardiologists had posterior subcapsular cataracts, citing radiation as the cause.6
The occupational effective dose limit to radiation workers is 20 mSv per year averaged over 5 years, and the dose limit for the eye has recently been reduced from 150 to 20 mSv a year to further protect against the rising number of radiation-induced cataracts.8 Recent evidence suggests that even protracted low-dose radiation exposure could be associated with leukemia, carotid artery atherosclerosis, and early vascular aging.9,10 Physicians need to keep the dose as low as reasonably achievable (ALARA),11 regardless of occupational dose limits. Radiation-attenuating materials, such as lead and lead-free materials (i.e. antimony and bismuth) have been used as a component of protective equipment to decrease the amount of radiation exposure. This includes aprons, glasses, gloves, and movable shields. With appropriate use, these will lower radiation exposure. Implementing radiation dose feedback may also have a role in reducing exposure. In this article, we review all the available tools to lower the radiation exposure dose to the operator during diagnostic, interventional, and electrophysiological cardiac procedures.
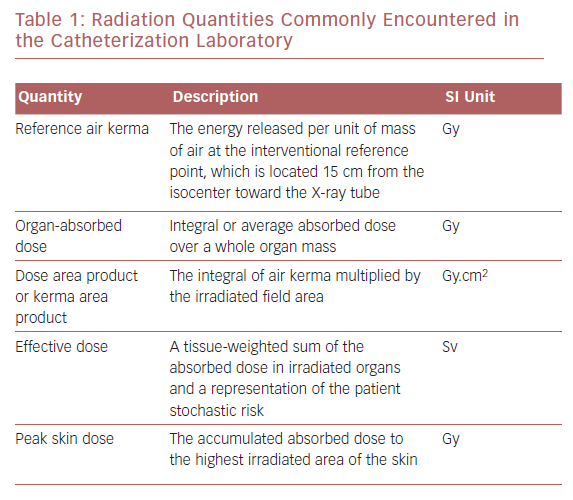
Basic Nomenclature in Radioprotection
Radiation exposure can be expressed as absorbed dose or kerma, which represents the energy of absorbed radiation by an organ or tissue per unit of mass, typically measured in milligray. Equivalent dose takes into account the radiation weighting factor relevant to the biologic effect of the absorbed radiation. In medicine, it is important to estimate the potential biologic effects on and the risk to an individual. The amount of radiation that an individual is exposed to is calculated by effective dose in millisieverts (Table 1). There are variables, including genetic and dietary variables, that affect radiation exposure and they vary between individuals. It is possible to combine effective doses of individual organs to obtain a global quantity (effective dose). Modern X-ray equipment detail information regarding the amount of radiation exposure to the patient by a measurement referred to as the kerma area product or dose area product (DAP).2
Magnitude of Radiation Exposure
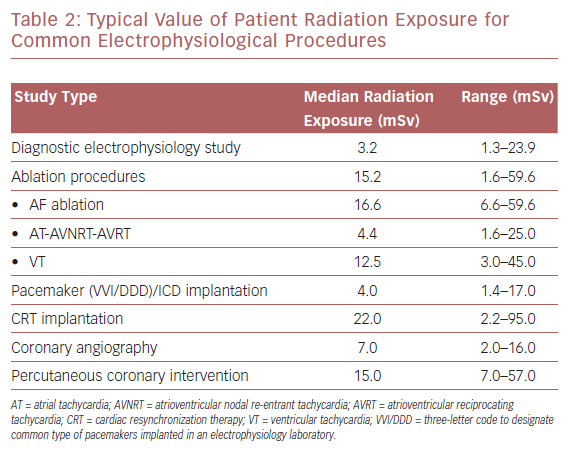
Modern cardiac interventional procedures, such as coronary angiography and percutaneous coronary intervention (PCI), have been shown to generate radiation exposure to 2–16 mSv and 7–57 mSv, respectively. Values for patient radiation vary from 3.2 mSv for a simple diagnostic electrophysiological study to higher values in more complex ablation procedures, such as in the treatment of AF, for which the reported median dose is 16.6 mSv (Table 2). Cardiac resynchronization device implantation is associated with the highest radiation exposure, with patients’ mean effective dose reported to reach 22 mSv per procedure.12,13
Estimating the amount of radiation dose to the physician in general is much more difficult due to the many variables involved from the energy source to the individual clinician. There is an impact of the hardware (detector size, image quality settings), procedure-specific factors (tube angulations, collimation, patient BMI), laboratory setup (use of protective lead lining, the position of the operator), and operator factors (use of personal protection).14
As a general rule, approximately 10–20% of photons are scattered. Adequate lead lining above and below the patient table is capable of blocking upwards of 80% of this scatter.15 A worst-case estimate of total physician dose in this setting would be 5% of the total estimated patient dose. The highest exposure during implantation of cardiac resynchronization devices was found to be 1.2 mSv (mean of up to 9 mSv) in one study.16
During structural interventions, the radiation exposure, as measured with DAP, was not found to be significantly greater than for single-vessel PCI.17 In structural heart disease laboratories, the radiation exposure to the operators performing transesophageal echocardiography has found to be at least as high as the primary operator.18 The amount of radiation exposure is significantly higher in endovascular interventions, especially with greater use of digital subtraction angiography runs, oblique angulations, and in thoracic procedures.19
The concept of the hybrid operating room involves radiation exposure to the surgical team and interventional cardiologists.20 The exposure can be minimized by various methods, as described later in this article.
Radiation exposure during pregnancy has been a cause for concern for many years. The maximum allowable total radiation dose in pregnancy is 500 mrem (100 mrem = 1 mSv), and the maximum monthly limit is 50 mrem.21 The effect on the fetus is based on gestational age. Exposure of up to 50 mrem in utero reduces the probability of malformation or cancer in live births from 95.93% to 95.92%, according to the National Council on Radiation Protection and Measurements.22
Methods of Reducing Radiation Exposure
Only procedures that have a valid scientific basis and are known to benefit a patient should be done. There needs to be responsible use of radiation-minimizing exposure time using the least amount of images. All physicians should work to the principle of ALARA.11
Physician Training in Radiation Protection
All staff should be trained to limit the dose of radiation and in radiation protection.12,23 Radiation protection and comprehensive training in the correct use of all available protection measures should be part of the curriculum of all trainees in interventional cardiology/specialized electrophysiology/device training programs.12
Careful attention should be paid to the correct use of protective shielding, and personal protection using an adequate lead apron, as well as the constant use of the radiation dosimeters in the correct position, should be implemented.24 Recognition of radiation exposure for both patient and operator/staff needs to be governed by the ALARA principle at all times, particularly for pediatric patients.11,25
Work Adaptations
Reducing Time of Radiation Exposure
The average procedure time for a diagnostic coronary angiogram is approximately 30 minutes and an interventional procedure (PCI or electrophysiology procedure/pacing) is between 90 and 120 minutes. However, the fluoroscopic and cine screening times are highly variable and depend on the nature of the procedure and the experience of the operator. The lower the amount of time spent in a radiation area, the lower the exposure.
Compared with acquisition cine imaging, fluoroscopy accounts for approximately 40% and 66% of the total air kerma dose in diagnostic catheterization and PCI, respectively.26 Fluoroscopy should be performed in short intervals, rather than continuously. The use of intravascular ultrasonography could also result in radiation dose reduction.26,27 Fluoroscopy storing is available in newer X-ray systems and also reduces the dose of radiation exposure.28 Virtual collimation, when available, reduces the radiation dose.28,29 Every effort should be made by the operating cardiologist to minimize fluoroscopy and cine screening time.
Increasing Distance of Operator from Radiation Beam
Increasing the distance from the radiation beam decreases the risk of exposure.30 Doubling the distance between the primary beam and operator reduces the exposure by a factor of four. Radiation exposure also varies according to the angle at which the camera is projected. Oblique views (left and right anterior oblique) and steep angulations increase radiation exposure, which are often employed to improve visualization. Angulations of 60 degrees give up to three times the operator dose than 30-degree angulations.14 The second operator or assistant is generally less exposed to radiation compared to the first operator, but is at greater risk than other staff in the operating room .
X-ray Angulation and Patient Positioning
Increasing the angulation of the imaging equipment (i.e. from 30 to 60 degrees) also increases radiation dose during both fluoroscopy and cine imaging.30 Proper positioning of both the patient and the operator is important for reducing radiation exposure. Radiation scatter is the primary mechanism of operator and staff exposure. Several factors can substantially affect radiation scatter, including the thickness of the X-ray beam, patient position, patient body surface area, access site, fluoroscopy and acquisition settings, filtration, shielding, and gantry angulation.29 Radial access increases exposure by reducing the distance between the X-ray beam and the operator; therefore, it is important to use manifold extensions and position the operator as close to the feet of the patient as possible.31 The height of the patient table can significantly affect scatter. The image receptor should be as close to the patient as possible.
Adjusting X-ray Settings and Fluoroscopy Rate
In most catheterization systems, the default setting for acquisition is 15 frames per second (FPS). During most interventional and electrophysiological procedures, the frame rate is kept at 7.5 FPS. By decreasing the fluoroscopy frame rate, radiation exposure is reduced by 30% and DAP by 19%.32 Most electrophysiological procedures can be done at 3.5 FPS, and often even at one FPS. During transseptal puncture and ablation near the atrioventricular node, higher frame rates are desirable.
Magnification and Collimation
Reducing magnification reduces the amount of radiation exposure. Newer X-ray systems offer features, such as storing fluoroscopy images, last image hold, and virtual collimation.29 There is a quasi-linear reduction in radiation dose with a reduction in the irradiated surface area.
Cine Acquisition
During cine, radiation exposure is five times higher than during fluoroscopy.27 The use of cine should be limited as much as possible. It is recommended that fluoroscopy images are stored in place of cine images.
Other available dose-reduction technologies include low pulse rate fluoroscopy, lower frame rate imaging, lower dose per frame, spectral beam filtration, and high X-ray beam energy.29
Robotic Percutaneous Coronary Intervention
Future modalities, such as the use of robotic systems to perform PCI, will result in a drastic reduction in operator exposure. The CorPath 200 robotic system was used in a study with eight patients and total radiation exposure was reduced by 97%.33,34
Remote-controlled robotic systems were developed to address the occupational hazards for interventionalists, such as chronic radiation exposure and orthopedic injuries, from long hours of standing while wearing heavy radiation protection gear. A prospective, multicenter study, Percutaneous Robotically-Enhanced Coronary Intervention Study (PRECISE) was carried out to compare robotic-assisted PCI to conventional PCI. The radiation exposure was 95.2% lower in the former.34
Non-fluoroscopic Guidance Systems on Electrophysiology
Non-fluoroscopic mapping systems commonly used in electrophysiology are Carto, Ensite–NavX, and MediGuide.35
In supraventricular tachycardia ablation, including Wolff–Parkinson–White syndrome, atrial flutter/atrial tachycardia, and AF, the Carto and Ensite-NavX systems reduced the fluoroscopy time and radiation dose.36–39 AF ablation can be done without radiation using the NavX system.40 MRI-guided catheter ablation offers minimal radiation with high resolution, but is still under development.
Radiation Shielding
Lead Caps
Brain tumors due to radiation have raised concerns regarding appropriate shielding of the head.3,4,41,42 The use of lead caps has been shown to reduce the dose to the head compared to ceiling-mounted lead shields.43,44 However, the weight of lead caps leads to neck and back pain. Lightweight lead-equivalent caps containing barium sulfate and bismuth oxide composite were found to reduce radiation dose by 90%; these caps weigh about 125 g and are comfortable to wear.45
Gloves
Hands are exposed to a significant amount of radiation (45–1,500 mSv per procedure) because they are unshielded and close to the radiation source.46 Lead gloves reduce the dexterity of operators’ hands. The use of lead-free radiation-attenuating latex gloves can reduce radiation by 58%.47 The best way to protect the hands is to shield them from the primary beam. Single-use unleaded radiation-protective gloves are recommended.
Eyewear
Catheterization laboratory staff and operating physicians are at high risk of radiation-induced cataracts. Studies have shown that using lead glasses lowers the dose of lens radiation by 98%.46–48 The use of non-lead glasses reduces the radiation dose by 36% compared to 87% with lead glasses. A lead acrylic face mask that protects the corners the eyes has been shown to reduce radiation exposure by 97%.49–51
Wearable Aprons
Lead aprons are effective in reducing the amount of radiation to the operator and are adapted as a standard of practice in all laboratories. The fit of the apron is important, as large gaps can increase radiation exposure to breast tissue in women.52 As they weigh a minimum of about 7 kg, their use can cause back problems.53 A custom-fit apron is recommended. Newer lead aprons made from lead composite or lead-free materials are about 20–40% lighter (around 4 kg) and offer similar protection to lead aprons.54 Lead-free bismuth-based materials provide good shielding, but are slightly less effective than standard lead aprons.54,55 Aprons should be quality checked annually to ensure that there are no cracks in the radioprotective layer. Aprons should be handled carefully and stored in purpose-designed racks to maintain the integrity of the radioprotective layer. Aprons should not be folded or creased during storage.
Thyroid Collar
The thyroid is a radiosensitive organ and has been linked to an increased risk of carcinogenesis with radiation exposure.56 It receives a significant amount of scattered radiation if it is not protected. Thyroid shields should be worn, and there should not be a significant gap between the thyroid shield and lead apron. They should be quality checked every year.
Ceiling-mounted Shields
Ceiling-mounted shields, if positioned correctly, reduce the radiation dose to the operator’s head and neck.15,48,57 The shield should be as close as possible to the patient to reduce scatter at the source.
Table Lead Skirts
The lower region of the operator receives the most exposure during the procedure. Protecting the lower trunk protects the reproductive organs, which are radiosensitive. Wearable aprons provide the majority of radiation protection, but using a table lead skirt reduces the radiation dose by 90%.15,58
Patient Drapes
Radiation drapes placed on top of the patient are important to prevent scatter radiation to the operator. RADPAD is a commercially available non-lead disposable shield that has been shown to reduce the operator radiation dose by 80% without increasing the radiation dose to the patient.59–61
Zero-gravity Radiation Protection System
The ceiling-suspended gantry system is available to support a walk-in lead-lined suit that eliminates the weight of lead aprons. These lead aprons have a 1-mm lead equivalency from the thyroid to groin, which is about twice the protection compared to standard lead aprons. It also provides 0.5 mm of protection where previously none was available.
Combined with table-side shielding, a ceiling-suspended lead screen reduces radiation to the primary operator by 76.8%, 81.9%, and 93.5% when placed close to the patient phantom, at the left side, and close to the primary operator, respectively, and reduces the radiation dose to the assistant by 70.3%, 76.7%, and 90%, respectively.62
EggNest Radiation Protection
The EggNest-XR system is a carbon fiber sledge platform with flexible shielding for radiation protection, which conforms to the patient’s body to allow 360 degrees of gantry motion. Significant reduction in radiation is achieved both to the patient and operator by this system. Compared to standard shielding, the EggNest reduces total room scatter radiation at all angiographic angles tested (average 92% reduction) and significantly reduces scatter radiation exposure at each of the six positions around the table at each angulation.63
Radiation Monitoring
Personal dosimeters are the gold standard to measure the dose of radiation to the operator. Two dosimeters are generally recommended to be worn: at the thyroid collar and under the protective apron. Eye dose can be measured by a dedicated eye lens dosimeter. The effective dose per interventional cardiologist varies from 1 to 4 mSv per year.29
Real-time monitoring of radiation exposure is a novel approach to reduce radiation exposure to modify the behavior of the operator. Bleeper Sv is a radiation detector that beeps at a rate proportional to radiation intensity. It helps to modify the behavior of the operator, and reduces radiation exposure according to the RadiCure study.64
The DoseAware System is designed to measure radiation exposure in real time and provides visual graphs to show per procedure, and daily and cumulative radiation exposure.65
Conclusion
Ionizing radiation is beneficial to neither patients nor staff in the catheterization laboratory. Every possible effort should be made to reduce the amount of radiation exposure, including scattered radiation.
Staff should use protective equipment to ensure that occupational radiation exposure is as low as reasonably possible. Unprotected areas, such as the head, neck, eyes, and hands, should also be protected using appropriate radiation shielding. A combination of tools, including procedural modifications, optimizing catheterization laboratory equipment, using a lower frame rate, and minimizing cine exposure and changing vascular access, will reduce the operator radiation dose. This should be done without compromising patient or therapeutic outcomes.
Staff training and education are important to achieve these goals. There should be a regular audit of each individual’s radiation exposure in the cardiac catheterization laboratory and each should be educated on how to minimize the dose of radiation.