Proprotein convertase subtilisin/kexin type 9 (PCSK9), discovered in 2003, is a circulating protein produced predominantly in the liver that plays a significant role in the recycling of LDL receptors (LDLRs).1,2 The LDLR, which normally recycles about 100 times in its lifetime, is the primary pathway for LDL-cholesterol (LDL-C) clearance from circulation. Plasma PCSK9 binds to LDLRs along with LDL-C, targeting the receptors for degradation, reducing their recycling and availability to clear LDL-C. Monoclonal antibodies (mAbs) bind to PCSK9 inhibiting the interaction of PCSK9 with LDLRs, preventing LDLR degradation and increasing the availability of LDLRs to enhance LDL-C clearance, resulting in reduction of LDL-C.1,2 In just 3 years following the publication of the first human studies with a PCSK9 mAb showing dramatic reductions in LDL-C, two agents (alirocumab and evolocumab) were approved by regulators and marketed worldwide for use as an adjunct to diet and maximally tolerated statin therapy in adult patients treated for clinical atherosclerotic cardiovascular disease (CVD) or heterozygous familial hypercholesterolemia (HeFH) who require additional LDL-C reduction.3–7 Evolocumab was also approved to treat patients ≥13 years with the rarer homozygous form of familial hypercholesterolemia (FH).5 In 18 months since approval results of a large and definitive outcome trial with evolocumab (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk; FOURIER trial) have demonstrated that the additional LDL-C reductions further reduce CVD events when added to statins.8 The initial concerns raised suggesting reductions in LDL-C below 50 mg/dl, or to low or very low LDL-C levels, would have little if any additional CVD benefit and may even be harmful by increasing the risk of hemorrhagic stroke, cognitive impairment, cataracts, or diabetes, have been proven to be misplaced on both efficacy and safety counts.9,10 These questions regarding benefit versus risk of greater LDL-C reductions and lower and lower LDL-C, which have plagued the use of all LDL-C reducing agents for decades, appear now to have finally been resolved, at least for drugs that enhance LDL clearance.8,11–13 Based on the extensive clinical trial evidence, PCSK9 inhibition with fully human mAbs clearly now has an important and routine role in CVD risk reduction. The major issues remaining for patients, physicians, and payers to enable widespread use and universal implementation of these very effective agents appears to be dosing frequency and cost.14,15 Hopefully, with agents currently in development requiring less frequent administration and more competitive pricing, if, and when, they come to the market in the next few years, these issues will be resolved.
Effect of PCSK9 Monoclonal Antibodies on LDLcholesterol Reduction and Other Lipoproteins
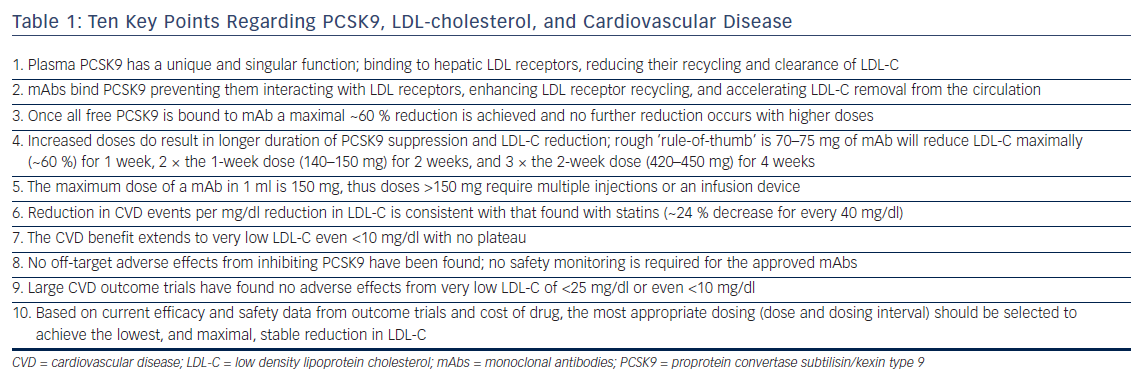
It is important to understand the fundamental difference between PCSK9 inhibitors and statins. All statins are dose limited by toxicity and, thus their LDL-C efficacy is defined not by potency but by the highest safest dose, which varies considerably between agents. The most potent statin ever marketed, cerivastatin, was reasonably effective but toxic at doses <1 mg/day, while rosuvastatin, which is safe at 40 mg/day, is the most efficacious statin marketed, reducing LDL-C ~55 %. This contrasts with PCSK9 mAbs where it was apparent in Phase I that once they bound all free PCSK9 a maximal ~60 % reduction in LDL-C was achieved.3,16 Higher doses produced no further reduction, or toxicity, but did result in longer duration of stable maximal LDL-C decrease (see Table 1). For alirocumab and evolocumab the relationship between dose and stable maximal LDL-C reduction follows a rough rule-of-thumb: 70–75 mg will reduce LDL-C 60 % for 1 week; twice the dose (140–150 mg) for 2 weeks; and three times the 2-week dose (420–450 mg) for 4 weeks.17,18 These doses administered less frequently will appear to produce less LDL-C reduction when LDL-C is measured just prior to the next dose (‘trough’ level) and results in what is referred to as a ‘saw-tooth’ effect where the LDL-C reductions vary over time.3,18–21 Another important difference between PCSK9 mAbs and other LDL-C lowering drugs is they have to be given by injection. A comfortable volume for an auto-injector is 1 ml, which holds a maximal of 150 mg of mAb, thus doses required for maximal stable LDL-C reductions for >2 weeks require multiple auto-injectors or a slow infusion device.22
PCSK9 inhibitors given in appropriate doses and dosing intervals uniformly reduce LDL-C ~60 % across patients on diet alone, low and maximal dose statin, or statin plus ezetimibe.23 They provide the same LDL-C response in patients with HeFH and non-FH and the response in HeFH is independent of underlying the LDL receptor mutation.23–25 In the rare patient with homozygous FH the LDL-C reduction with the approved dose of evolocumab, 420 mg monthly, is ~30 %, half that of HeFH and non-FH patients, and the response is heavily dependent on the underlying genetic mutations.26 A number of studies have shown that statin-adverse patients tolerate PCSK9 mAbs well.27,28 In addition to LDL-C reductions PCSK9 mAbs produce expected parallel reductions in non-HDL-C and apolipoprotein B, and small increases in HDL-C. Unlike statins and ezetimibe PCSK9 inhibition results in a robust 25–30 % decrease in lipoprotein(a) but does not reduce high-sensitivity C-reactive protein (hsCRP).29,30
PCSK9 Monoclonal Antibodies and Cardiovascular Disease Risk Reduction
Despite robust reductions in LDL-C with statins, which were first approved for use in 1988, it took another 7 years until the results of the Scandinavian Simvastatin Survival Study (4S) trial to provide evidence of CVD benefit.31,32 This contrasts with PCSK9 mAbs where data from Phase III with evolocumab and alirocumab strongly suggested CVD benefit.24,33 The encouraging results were fully validated by FOURIER, the largest and shortest duration CVD outcome trial for a LDL-C lowering agent, which achieved a 15 % reduction (hazard ratio [HR] 0.85; 95 % CI [0.79–0.92]; p<0.001) in the primary endpoint, a composite of cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization.8 The secondary ‘hard endpoints’ of CVD death, definitive MI, and stroke were reduced 20 % (HR 0.80; 95 % CI [0.73–0.88]; p<0.001). Some, especially those in the media and financial world, expressed “disappointment” in the magnitude of reduction, which was likely based on a lack of understanding of the trial’s stopping rules along with the raised expectations from the two small exploratory or post hoc studies.14,15,34 FOURIER was designed to terminate when at least 1,630 patients had experienced the key secondary endpoints, which was expected to provide 90 % power to detect a relative reduction of at least 15 %. Based on an assumption of a 2 % per year event rate in the placebo arm, it was anticipated that the 27,500 patients would be treated for a median of about 43 months.35 However, the key secondary endpoint rate was nearly double projections with the trial achieving 1,829 endpoints after a median of 26 months requiring termination, and the reduction in the primary endpoint exceeded the preset HR of 0.8 5 % (p<0.001).8 Concern has been expressed as there was no difference in CVD death, which may be related to the short duration of the trial; however, the two serious, debilitating and costly events of MI and stroke were reduced by 27 % and 21 % (p<0.001 and p<0.01), respectively.8 Furthermore, as emphasized by the FOURIER investigators, the reduction in CVD events increased progressively with duration of treatment and at 3 years were similar to that seen with statin therapy.8
Cardiovascular Disease Benefit and Very Low LDLcholesterol
Preliminary evidence of continuing CVD benefit down to very low LDL-C of <25 mg/dl came from a post hoc analysis by Ray et al. of CVD events in 10 Phase III trials with alirocumab.36 They showed LDL-C reduction from a mean of 50 mg/dl to a mean of 25 mg/dl (i.e. half the patients had LDL-C <25 mg/dl) resulted in as much CVD reduction as reducing LDL-C from a mean of 75 mg/dl to a mean of 50 mg/dl.36 Ray et al. concluded that the relationship seen in statin trials between reductions in LDL-C and CVD of every 40 mg/dl decrease in LDL-C resulted in a 24 % reduction in CVD events, was consistent down to the lowest LDL-C achievable in these trials.36 Further evidence was provided from an exploratory post hoc analysis in patients with baseline LDL-C <70 mg/dl in the Global Assessment of Plaque Regression With a PCSK9 Antibody as Measured by Intravascular Ultrasound (GLAGOV) trial.37 Compared with the placebo group (mean LDL-C 70.6 mg/dl), those treated with evolocumab (mean LDL-C of 24 mg/dl) had significantly greater reduction in percent atherosclerosis volume (−1.97 % versus −0.35 %; p<0.001).37
Using a Locally Weighted Polynomial Regression analysis, GLAGOV showed a linear relationship between on-trial achieved LDL-C level and atheroma regression down to a LDL-C of 20 mg/dl.37 Definitive evidence was provided in the recent FOURIER trial where the median LDL-C in the evolocumab group was only 30 mg/dl, 42 % of subjects had LDL-C <25 mg/dl and 25 % <20 mg/dl.8 In a subsequent prespecified secondary analysis from FOURIER, Giugliano et al. reported in greater detail the relationship between LDL-C and the primary and secondary CVD outcomes as well as 10 prespecified safety events.13 LDL-C, measured by the ‘gold standard’ ultracentrifugation technique due to inaccuracy of calculated LDL-C at low LDL-C levels, was <20 mg/dl in 2,669 patients, 20 mg/dl to <50 mg/dl in 8,003, 50 mg/dl to <70 mg/dl in 3,444, 70 mg/dl to <100 mg/dl in 7,471, and ≥100 mg/dl in the remaining 4,395 patients. The relationship between LDL-C and the primary and secondary CVD endpoints extended to a LDL-C of <10 mg/dl (the bottom 1st percentile), p=0.0012 for the primary endpoint, p=0.0001 for the secondary endpoint.13
PCSK9 Monoclonal Antibodies and Safety
Despite the need for regular subcutaneous administration both fully human mAbs, alirocumab, and evolocumab have good tolerability and adherence. Based on the data from the clinical development programs in >10,000 patients treated with these two mAbs for 3+ years, no specific or serious clinical or laboratory adverse events were found. As a result regulatory authorities required no specific safety monitoring in the label for either agent, which is unusual for an entirely new class of lipid lowering agents.4–7 This has been supported by safety data from larger and longer trials such as FOURIER and Studies of PCSK9 Inhibition and the Reduction of Vascular Events (SPIRE).8,12 Mild injection site reactions and antidrug antibodies have been reported with the approved mAbs but no physical, psychological, endocrine, or reproductive abnormalities or ‘off-target’ effects have been observed. However, development of neutralizing antibodies that reduced or eliminated LDL-C lowering, along with increased rate and severity of injection site reactions with bococizumab, a not-fully human PCSK9 mAb, resulted in termination of its development.38 Some caution regarding longer-term use is necessary because, as with statins, it may take decades of therapy in many tens of thousands of patients to detect more subtle, or agent-specific, sideeffects not readily apparent from relatively short-term trials. Inhibition of plasma PCSK9 itself has not be associated with adverse effects but Mendelian randomization studies suggest PCSK9 loss-of-function variants are associated with roughly the same effect as loss-of-function variants in 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMGCR) in terms of the risk of diabetes per unit decrease in the LDL-C.39,40 An analysis by Ference et al. found the increased risk of diabetes for HMGCR and PCSK9 loss-of-function variants were independent and additive, but was confined to people with impaired fasting glucose levels.39 As documented from extensive clinical trial data with statins, the risk of diabetes from Mendelian randomization studies was less than the protective effect against cardiovascular events, which account for the majority of mortality and morbidity in diabetics.
Do Very Low LDL-cholesterol Pose Safety Concerns?
For more than 40 years, going back to clofibrate and bile acid sequestrants, reducing LDL-C and lower LDL-C levels have been postulated to be “harmful” with concerns ranging from cancer to suicide.41 Many of the associations have been based on faulty interpretation of epidemiological data or post hoc analysis of adverse events in clinical trials that were drug or mechanism specific. Actual relationship to LDL-C levels has never been validated and repeatedly dispelled by additional clinical trial evidence, and decades of routine use of more and more efficacious LDL-C lowering agents and lower and lower LDL-C levels.11,42 However, this has not prevented similar concerns to be raised with PCSK9 mAbs, which when added to statins are able to routinely reduce LDL-C to levels rarely achieved in the past.9,10,43 These latest concerns focused on hemorrhagic stroke, cognitive impairment, diabetes, and cataracts.11–13,43 As discussed, Mendelian genetic variant studies demonstrate a small increased risk of diabetes in a subgroup of people with already impaired glucose tolerance associated with both HMGCR and PCSK9 variants.39 However, the risk was not related to low or very low LDL-C but to the reduction of LDL-C from any level, which has been confirmed from clinical trial data with statins and is now included in their labeling. The concern of cataracts emanated from a post hoc analysis of the alirocumab pooled Phase II and III trials by Robinson et al., which reported an increase in self- or physicianreported cataracts with alirocumab in groups achieving LDL-C <25 mg/dl or <15 mg/dl.43 However, the analysis contained a number of substantial flaws that cannot be eliminated by statistical manipulation: at baseline these groups had 30–50 % more diabetics, worse diabetes control as judged by hBA1c levels, and were older than groups with LDL-C >25 mg/dl or placebo.43 The analysis also used calculated LDL-C, by Friedewald formula, to select the LDL-C cut points, which underestimates true LDL-C by approximately 30 % and misclassifies about one-third of subjects with levels <25 mg/dl.44 The cataract concerns were rapidly put to rest by the recent analysis by Giugliano et al. of very low LDL-C levels in FOURIER and further supported by those from the bococizumab SPIRE trial.12,13 In SPIRE, a prespecified safety analysis assessed new cataract development in 6,285 bococizumab-treated patients and LDL-C <25 mg/dl compared with 7,259 treated with bococizumab >25 mg/dl and 13,967 placebo-treated patients. Not only was the rate of 0.9 per 1,000 patient years of treatment in the very low LDL-C group not increased but was lower than the rates of 1.3 and 1.1 per 1,000 patients in the LDL-C >25 mg/dl and placebo groups, respectively.12 A lack of any relationship between hemorrhagic stroke and low LDL-C levels was initially reported by Wiviott et al. from the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT) trial where they found no hemorrhagic stroke in patients with LDL-C <40 mg/dl.42 Further evidence of no increased risk came from the nearly 1,000 patients in the Improved Reduction of Outcomes: Vytorin Efficacy International (IMPROVE-IT) trial treated with statin or statin plus ezetimibe for a median of 6 years who achieved LDL-C <30 mg/dl and had a lower rate of hemorrhagic stroke compared with the groups >30 mg/dl.11
The safety analysis from FOURIER and SPIRE trials also found no relationship between hemorrhagic stroke, cognitive function, or cataracts, and very low LDL-C levels.12,13 Cognitive impairment was assessed in a prospective substudy (Evaluating PCSK9 Binding Antibody Influence On Cognitive Health in High Cardiovascular Risk Subjects; EBBINGHAUS) as part of FOURIER, which found no difference compared with placebo.45 The analysis by Giugliano et al., which focused on very low LDL-C <20 mg/dl examined 10 prespecified safety endpoints, including cataract-related adverse events, hemorrhagic stroke, neurocognitive adverse events, and new-onset diabetes mellitus.13 They reported no significant association between achieved LDL-C and any of the safety concerns, and when taken together with the benefit of decreased CVD events concluded that the data from FOURIER supported further LDL-C lowering in patients with CVD to well below current recommendations.13 The decision by regulators to not require safety monitoring for low LDL-C for either alirocumab or evolocumab is now further supported by the FOURIER and SPIRE trials. On the basis of all the evidence generated in regard to benefit/risk related to very low LDL-C over the last decade, it is long past the time for consensus guidelines by bodies such as American College of Cardiology and American Heart Association to eliminate the arbitrary, data deficient, suggested lower ‘safe’ LDL-C of 40 mg/dl, which appear in guidelines and laboratory reports and only serve to generate anxiety for patients and clinicians when LDL-C is now reduced below this level.9 The data generated from PCSK9 mAbs strongly supports that, just as for hsCRP, no lower limit for LDL-C should be specified based on the available evidence that lower is better.
Alternative Approaches to Monoclonal Antibodies for Inhibiting or Reducing PCSK9
The majority of PCSK9 is produced in hepatocytes, and reducing synthesis with small interfering RNA (siRNA) targeted to the liver provides a selective mechanism to silence the translation of PCSK9 messenger RNA.46 Inclisiran, a chemically-synthesized siRNA molecule, produces sustained hepatocyte specific PCSK9-specific RNA silencing. In a Phase II multicenter, double-blind, placebo-controlled trial patients were randomized to either a single subcutaneous dose of placebo or 200, 300, or 500 mg inclisiran, or two doses at 90-day intervals of placebo or 100, 200, or 300 mg of inclisiran. The investigators recently reported serum PCSK9 reductions of 70–80 %, and decreases in LDL-C of approximately 50 % at 90 days post-dosing with 300 mg.47 Inclisiran is now entering Phase III trials and is anticipated that dosing frequency will be two or three times a year.
A second approach, much earlier in clinical development, is a vaccine that generates antibodies against PCSK9 to provide prolonged PCSK9 suppression and LDL-C reduction. A peptide (AT04A, AFFITOPE®, AFFiRiS AG) designed to mimic the N-terminal epitope of the mature human and mouse homolog PCSK9 protein (amino acids 153–692), and formulated into a vaccine, has been reported to be effective in a rodent model. The agent is currently in Phase I trials.48
Conclusion
PCSK9 inhibitors are the most well understood, targeted, specific, and effective LDL-C lowering agents yet developed and when added to statins in patients with CVD result in very low LDL-C and significant additional reduction in CVD events with good tolerability and safety (see Table 1). The ability of these agents to safely reduce LDL-C in patients who need further LDL-C reduction despite maximal tolerated statin therapy should result in their rapid incorporation into the prevention regimen for CVD patients to help minimize, if not eliminate, the component of atherosclerosis related to LDL.