Dyspnea, palpitations, edema, and fatigue are common symptoms during pregnancy. For women with congenital heart disease (CHD), it may be difficult to discern whether symptoms are due to normal pregnancy or underlying cardiac disease. Although most women with CHD tend to experience successful pregnancies, morbidity and mortality are significantly increased with more complex CHD lesions.1–6 Women with CHD are at risk for arrhythmias, heart failure (HF), thromboembolic complications, and preeclampsia.1–6 Risks to the fetus also exist, including premature birth, small for gestational age (SGA), neonatal death, and the risk of recurrence of CHD.1–3 Many of these maternal complications can be treated, and perhaps even anticipated. As the number of maternal CHD pregnancies rises,4 clinical cardiologists should understand the complications with specific CHD conditions and know when to refer women for more specialized care. This review discusses common clinical case scenarios seen by the general cardiologist.
Physiology of Pregnancy
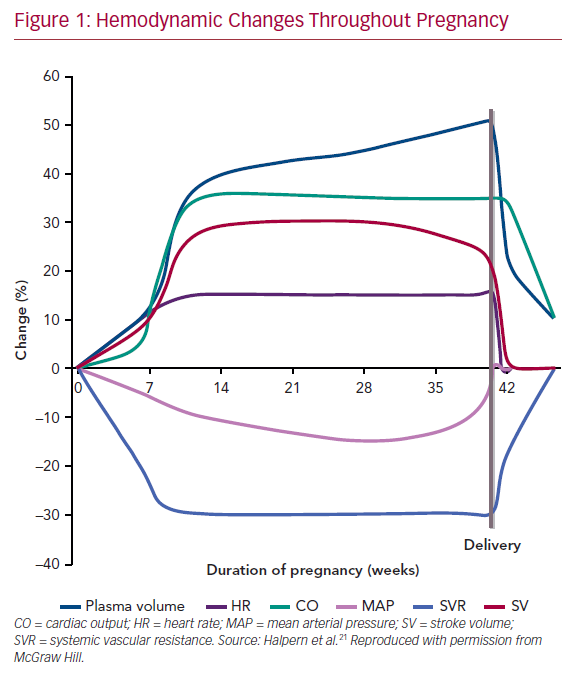
Pregnancy encompasses rapid and profound anatomical and physiological changes (Figure 1). Healthy women compensate for significant hemodynamic changes during pregnancy. However, in women with underlying CHD, the hemodynamic adaptations of pregnancy may cause further burden to both the mother and fetus. Conception initiates physiological adaptations, which persist for several months postpartum.7,8 Estrogen, progesterone, and activation of the renin–angiotensin–aldosterone system results in plasma volume expansion. A disproportional stimulation of erythrocyte mass occurs compared with volume expansion, resulting in dilutional anemia. Further modulation of hormones produces systemic vasodilation to accommodate this volume expansion. As the placenta matures, systemic vascular resistance decreases until the third trimester, during which it rises slightly. Concurrently, pulmonary vascular resistance decreases to allow for the increase in pulmonary flow. A fall in blood pressure occurs until the third trimester, after which blood pressure begins to rise gradually. Importantly, cardiac output increases as much as 50% by around 24 weeks to allow for these hemodynamic changes. The early increase in cardiac output is due to increasing circulating volume. Later, the output is augmented by increased heart rate. Importantly, pregnancy is a hypercoagulable state, increasing the risk of thromboembolic complications fivefold by the third trimester and peaking early postpartum.9 Multiple gestations can intensify these consequences.
Labor and delivery cause marked increases in heart rate, cardiac output, and central venous pressures.7,8 Cardiac output increases 60–80% immediately postpartum due to the uteroplacental autotransfusion and vena cava decompression. Changes in hydrostatic and colloid osmotic pressure increase the risk of pulmonary edema at the time of delivery and immediately postpartum. Within the next 24–72 hours, heart rate and cardiac output fall. By 2 weeks postpartum, most of the hemodynamic changes return to their prepregnancy state; however, it may take up to 6 months for cardiac remodeling to resolve. Due to the significant and rapid hemodynamic changes of labor and in the early postpartum period, women with underlying cardiovascular disease are at increased risk of decompensation during delivery and postpartum, especially within the first 48 hours.
Medications
Cardiovascular medications are frequently necessary during pregnancy in women with underlying heart disease. The risks and benefits to both the mother and fetus must be considered when determining the use and safety of medications during pregnancy.7,8,10 Exposure to medications during the first 2 weeks after conception can result in fetal demise, whereas teratogenicity usually occurs between 4 and 12 weeks of gestation. An extensive review of the safety of medications for cardiovascular disease during pregnancy has been published previously.10 Beta-blockers are the most widely used cardiac medication during pregnancy. With the exception of atenolol, beta-blockers have a favorable safety profile; however, they are associated with an increase in SGA births, neonatal bradycardia, and hypoglycemia. Calcium channel blockers, nifedipine for hypertension and verapamil for arrhythmias, are the preferred agents. Calcium channel blockers can be associated with prematurity, intrauterine growth restriction (IUGR), fetal bradycardia, and suspected neonatal seizures if used in the third trimester. Pulmonary edema necessitates judicious diuresis with loop diuretics, weighing the risk of oligohydramnios, decrease in placental perfusion, and fetal electrolyte abnormalities. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, direct renin inhibitors, and spironolactone are all contraindicated during pregnancy and should be discontinued prior to conception. Hydralazine and oral isosorbide dinitrate may be used as substitutes for afterload-reducing medication. Of note, captopril, benazepril, and enalapril may be considered during lactation. Amiodarone is associated with fetal hypothyroidism and IUGR unrelated to duration or dose, and therefore should be reserved for life-threatening refractory arrhythmias. Instead, adenosine, digoxin, or lidocaine, and, used with caution, sotalol, flecainide, or propafenone may be considered for arrhythmia management. Drugs such as endothelin receptor antagonists, statins and direct oral anticoagulants are contraindicated during pregnancy and appropriate alternatives should be discussed.
Preconception counseling regarding the risks and benefits of anticoagulation including warfarin and heparin by an experienced provider is essential because all pose an increased risk of gestational complications.7,8,10 Warfarin crosses the placenta and is associated with embryopathy, miscarriage, and stillbirth, with increasing complications at doses >5 mg. Despite these risks in women with mechanical valve, warfarin is associated with the lowest risk of adverse maternal outcomes and should be recommended as per guidelines. Low-molecular-weight heparin (LMWH), which does not cross the placenta, is associated with the lowest risk of adverse fetal outcomes, but higher maternal risk in those with mechanical valves. LMWH is the preferred agent for all other indications. In addition, frequent monitoring of levels, conversion between medications as an inpatient, and a planned delivery are necessary.
Cases
Case 1: Prepregnancy Evaluation of a Woman with Bicuspid Aorta with Moderate Aortic Stenosis and Repaired Coarctation
Prepregnancy counseling and evaluation is essential in woman with CHD, especially those at highest risk.7,8 Unfortunately, most women do not receive appropriate counseling and optimization of their CHD. Without appropriate prepregnancy counseling and optimization of their CHD, women have double the risk of maternal mortality and HF.2
One of the first steps in counseling a patient with CHD is to determine their risk with the modified WHO (mWHO) classification.8 Women with mWHO I, such as those with mild pulmonary stenosis or a repaired simple atrial septal defect (ASD), have a small increase in morbidity and no increase in mortality compared with the general population. Conversely, mWHO IV, such as woman with symptomatic severe aortic stenosis or severe systemic ventricular dysfunction, have the highest risk of maternal complications, with cardiac event rates of 40–100%. mWHO IV individuals should be counseled against pregnancy and, if pregnancy occurs, discussions regarding termination are essential. Women with stenotic bicuspid aortic valve and repaired coarctation without significant residual narrowing or aneurysm are classified as mWHO II–III and have an intermediate risk of morbidity and mortality with a cardiac event rate of 10–19%. Other risk scores, such as CARdiac disease in PREGnancy (CARPREG I and II) and Zwangerschap bij vrouwen met een Aangeboren HARtAfwijking (ZAHARA), further assist in stratifying a patient’s risks (Table 1).11–13 The latter risk score is focused on CHD.
The next step in risk stratification is to determine the anatomic and physiological complexity of the defect.7,8 Reviewing prior surgical and catheterization reports assists in understanding the underlying anatomy and potential complications. Echocardiography further determines the underlying anatomy and spectrum of CHD (e.g. from mild to severe aortic stenosis). However, an echocardiogram may not reveal the true extent of an aortic aneurysm or stenosis of the aorta. Therefore, women with coarctation of the aorta (CoA) should undergo an MRI or ECG-gated CT scan prior to pregnancy. If the woman is pregnant at the time of presentation, an MRI without gadolinium after the first trimester may be performed. Women with hemodynamically significant CoA are advised to undergo stent placement prior to pregnancy. In addition to anatomic evidence seen on imaging, significant CoA is defined as: upper or lower extremity resting peak-to-peak gradient >20 mmHg or mean Doppler systolic gradient >20 mmHg; upper or lower extremity gradient >10 mmHg or mean Doppler gradient >10 mmHg plus either decreased LV systolic function, aortic regurgitation or with collateral flow.7,8,14
Aneurysms may also be seen on imaging, and careful indexing of the absolute aortic dimensions to the patient’s size (i.e. body surface area) is crucial to clinical decision making.7,8,14 Patients with smaller body habitus may be erroneously judged to have normal aortic dimensions by their absolute measurements. Thus, individuals with a smaller body habitus, such as patients with Turner syndrome who become pregnant, commonly by assisted reproductive therapy techniques or by being Turner mosaic, are advised to undergo aortic replacement when the aorta exceeds 25 mm/m2. Non-Turner patients with bicuspid aortic valves are advised to undergo aorta replacement when the absolute aortic size exceeds 50 mm or 27 mm/m2. In those with genetic aortopathies, such as Marfan syndrome, replacement at smaller sizes is advisable. Even with ascending aortic root replacement, women with aortopathies remain at risk of type B dissection. In addition, intracranial aneurysm in adults with CoA occur, and women should be screened for as per guidelines.
Prepregnancy exercise testing is also an important screening tool because many women under-report or under-recognize the degree of their limitation.7,8,14 Exercise testing reveals symptom burden and identifies high-risk exercise test features, including reduced exercise capacity (peak oxygen consumption, VO2), abnormal blood pressure response, ischemic changes, or arrhythmias. If a woman is found to have symptomatic severe aortic stenosis, asymptomatic severe aortic stenosis with left ventricular (LV) dysfunction, or high-risk exercise test features, she should be advised against pregnancy and referred for valve replacement as per guidelines. Further, women with hemodynamically significant CoA are advised to undergo stent placement prior to pregnancy, and aneurysms should be repaired at sizes as specified above.
Woman with aortic stenosis should be counseled on and assessed for HF, angina, and syncope.7,8 As pregnancy progresses, the decline in afterload and increase in volume increases the aortic gradient and limits the ability to augment cardiac output appropriately. These changes in hemodynamics increase the risk of HF, arrhythmias, and angina, even if not present prior to conception. If a complication arises, such as HF, medical management with diuretics may be required with caution, given fixed cardiac output, and concern for fetal hypoperfusion. If these attempts fail, patients should be evaluated for possible balloon aortic valvuloplasty or surgery.
Women with coarctation are at increased risk of hypertension disorders, including preeclampsia.7,8 Low-dose aspirin should be considered after 12 weeks of gestation to decrease the risk of severe preeclampsia.15 Hypertensive medication should be appropriately adjusted prior to pregnancy and titrated during pregnancy. Beta-blockers are the first-line choice to treat hypertension.7,8,10 Aggressive blood pressure lowering should be avoided in women with residual or native coarctation because this may result in uterine hypoperfusion. Rarely, catheter-based interventions during pregnancy are required for maternal or fetal compromise. If a catheter-based intervention is deemed necessary, caution should be used because hormonal changes of pregnancy and the hyperkinetic circulation increase the risks of dissection and dilation of the aorta.
After appropriate counseling and optimization, if pregnancy occurs vaginal delivery with an expedited second stage and regional anesthesia should be considered in women with asymptomatic stenosis or aneurysm 40–45 mm.7,8 In those women with symptomatic aortic stenosis, aortic aneurysm >45 mm, or progression of an aneurysm, a cesarean section is indicated. A cesarean section can be considered in women with unrepaired coarctation and aneurysms.
Case 2: Woman with a Newly Diagnosed Murmur at 24 Weeks Found to have an Atrial Septal Defect With Mildly Dilated Right Ventricle and Normal Systolic Function
Atrial septal defects (ASDs) are one of the most common CHDs in pregnancy. ASDs may be newly diagnosed in pregnancy because the hemodynamic changes exaggerate right ventricular (RV) volume and may unmask an undiagnosed ASD.7–9 Unrepaired (mWHO Class II) or repaired (mWHO Class I), ASDs are usually well tolerated in pregnancy unless associated with cyanosis or pulmonary hypertension. Women are at a <5% risk of arrhythmias, which occur more frequently in those with unrepaired shunts or those with shunts repaired at older ages. There is also a small risk of paradoxical emboli; thus, any signs of deep venous thrombosis should be investigated. Aspirin should be considered after 12 weeks because there is an increased rate of preeclampsia. Other complications include SGA and higher fetal or perinatal mortality. Rarely will ASD closure be required during pregnancy unless cyanosis occurs without significantly elevated pulmonary vascular resistance. Similarly, women with repaired small ventricular septal defects (VSDs) or small patent ductus arteriosus without an increase in pulmonary vascular resistance tolerate pregnancy well. Vaginal delivery is usually well tolerated with a consideration for IV air filters to prevent air embolisms.
If an ASD or other shunt results in Eisenmenger syndrome (i.e. irreversible pulmonary vascular disease with reversal of the shunt direction and cyanosis; mWHO Class IV), maternal mortality has been reported to be as high as 20–50%.16,17 These individuals should be counseled strongly against pregnancy and, if needed, should undergo early termination.8 If resting arterial oxygen saturation is <85%, the likelihood of a live birth is 12%. Pregnant women with Eisenmenger syndrome or cyanotic heart disease should be referred to advanced CHD centers for further care given their significant morbidity and mortality risk.
Case 3: Palpitations in a Pregnant Woman with Tetralogy of Fallot Status, Severe Pulmonary Regurgitation, Dilated and Hypokinetic Right Ventricle
Eight per cent of women with repaired tetralogy of Fallot (TOF), mWHO II, endure cardiac complications such as arrhythmias and HF during pregnancy.8,18 Given the increased risk of arrhythmias, woman who complain of palpitations should be evaluated with a Holter or event monitor. Extra volume load, enhanced adrenergic receptor excitability, and surgical scars all increase a woman’s risk of both atrial and ventricular arrhythmias.19 When AF occurs, rhythm control with cardioversion is preferred over rate control with beta-blockers with concurrent anticoagulation.7,8 Electrical cardioversion should be performed in women with hemodynamic instability.
In addition to arrhythmias, RV dilation and HF can occur in women with repaired TOF, especially in those with underlying severe pulmonic regurgitation with RV dysfunction, left ventricular dysfunction, and pulmonary hypertension.1,14 Therefore, prior to pregnancy, in addition to an echocardiogram, cardiac MRI is recommended to evaluate pulmonary regurgitation, RV dilation, and ventricular function in women with repaired TOF.8,14 Optimal timing of pulmonary valve replacement prior to pregnancy should be as per the American College of Cardiology/American Heart Association guidelines.14 As with all women with CHD, close monitoring throughout pregnancy is required because it is difficult to distinguish symptoms and signs of normal pregnancy from those that may reflect hemodynamic compromise of HF. Screening with N-terminal pro B-type natriuretic peptide (NT-proBNP) at 20 weeks of gestation has a good negative predictive value regarding cardiovascular events.20 Echocardiograms should be performed at a minimum of every trimester.8 If RV HF occurs, medical management with diuretics is indicated. Very rarely, trans-catheter pulmonary valve implantation is required in those not responding to medical management with severe pulmonary regurgitation and RV HF. Thromboembolism and endocarditis have seldom been reported.
In addition to maternal echocardiograms, woman with TOF and other forms of CHD should be offered a fetal echocardiogram at 18–22 weeks of pregnancy because it identifies 45% of congenital cardiac malformations.7,8 Parents should also be offered 22q11 deletion testing and genetic counseling. In those whose disease arises de novo, CHD recurrence in offspring is 3–5%.
Given the risks associated with pregnancy in TOF, a clear plan for labor, delivery, and postpartum care should be developed by the end of the second trimester and distributed to all members of the care team.7,8 As with all complex congenital heart diseases, careful cardiac monitoring is recommended because the odds of an adverse maternal cardiac event, including HF, arrhythmia, and thromboembolic events, during delivery are 2.4- to 27.6-fold higher for women with than without CHD.3 Obstetric complications are also higher in these women, including preeclampsia, preterm delivery, hemorrhage, and placental abruption. Higher rates of comorbidities, such as pulmonary hypertension, coronary artery disease, conduction and rhythm disorders, mental health, neurological, and pulmonary conditions, accompany women with CHD and require consideration during delivery.
Vaginal delivery should be encouraged in women with CHD because it has less blood loss and lower risks of infection, venous thrombosis, and embolism.7,8 Elective cesarean sections have no maternal benefit and result in earlier delivery and lower birth weight. Therefore, cesarean sections are reserved for obstetric indications and specific cardiac indications, including current warfarin therapy, dilated aortic root, aortic dissection, and intractable HF. An early epidural should be carefully titrated during labor and delivery. An epidural minimizes pain and intrapartum fluctuations in cardiac output, but can cause systemic hypotension in those with obstructive valve lesions or diminished ventricular function. Fluids should be meticulously titrated. Hemorrhage causing tachycardia and decreased stroke volume is poorly tolerated, especially in women with preload-dependent hearts. Thus, to prevent hemorrhage, a slow infusion of oxytocin may be safely used, whereas vasodilatation and pulmonary vasoconstriction can occur with an intravenous bolus.
Due to the rapid and significant hemodynamic adaptations, postpartum women with complex CHD should be closely monitored, including telemetry for a minimum of 24–48 hours. After discharge, women should be seen intermittently as outpatients for at least 6 months.
Case 4: Postpartum Heart Failure in a Woman With Transposition of the Great Arteries Status After Repair
Many women of reproductive age with dextro transposition of the great arteries (D-TGA) have undergone the atrial switch operation (i.e. Mustard or Senning), with redirection of the systemic and pulmonary venous return at the atrial level resulting in a systemic RV (mWHO III).7,8,14 Pregnancy can be relatively well tolerated if there is less than moderate impairment of RV function or moderate tricuspid regurgitation. However, the systemic RV is prone to failure and worsening tricuspid regurgitation in the setting of the hemodynamic changes of pregnancy, requiring HF therapy with diuretics.7,8 In addition, atrial baffles, as part of the atrial switch operation, may narrow or leak, resulting in shunting with the potential for systemic cyanosis and paradoxical emboli. Further, women are at risk of sinus node dysfunction and atrial or ventricle arrhythmias. Therefore, monthly or bimonthly surveillance with echocardiograms and arrhythmia monitoring is necessary during pregnancy and postpartum.
Since the late 1980s, an alternative to the atrial switch has been an arterial switch, which is now performed more frequently.14 The surgery for an arterial switch consists of transection of the pulmonary artery and moving it anteriorly (LeCompte maneuver), and translocation of the coronary arteries to the neoaorta (previous pulmonary root). Long-term complications include stenosis of the great arteries or coronaries at the reimplantation sites, and neoaortic root dilatation. A prepregnancy evaluation of ischemia, supravalvular obstruction, dysfunction, and aneurysm of the neoaortic valve is recommended.7,8 If there is an aneurysm of the neoaorta, echo surveillance during pregnancy is advised. In the absence of residual structural abnormalities, women with a prior arterial switch procedure do well; however, data are limited.
Systemic RV also occurs in women with congenitally corrected transposition (levo transposition of the great arteries [L-TGA]). L-TGA can be associated with pulmonary stenosis and VSD precipitating surgical intervention (mWHO III).7,8,14 Cardiac complications can include heart block, atrioventricular valve regurgitation, and HF. Importantly, as many as 10% will have an irreversible fall in RV function during pregnancy. Therefore, echo surveillance of the systemic RV function should be performed every 4–8 weeks.
Women with systemic RV with either d-TGA with atrial switch or L-TGA with underlying ventricular dysfunction, left-sided obstructive lesions, or Eisenmenger syndrome are at highest risk for HF.1,6 Ventricular dysfunction, even if mild, can be further impaired due to increasing demands of pregnancy. Pregnancy may worsen systemic tricuspid regurgitation, which may persist postpartum. In women with structural heart disease, preeclampsia can also increase the risk of developing HF. Symptoms of HF may occur during pregnancy or up to 6 months postpartum, warranting vigilant postpartum evaluation. If RV dysfunction or worsening tricuspid regurgitation occurs, it may be irreversible, and future pregnancies should be discouraged with any dysfunction beyond the mild range. When HF occurs, management is guideline-directed therapy.7,8,14
Conclusion
Advances in medicine have resulted in improved survival in women with CHD who desire pregnancies. General cardiologists are now being tasked with caring for these women, and it is important to have a thorough understanding of these unique hemodynamics and the potential complications that can arise. Although the majority of these women can expect to tolerate pregnancy well, it is imperative that physicians engage in prepregnancy counseling and remain vigilant for issues such as HF, arrhythmia, and thromboembolic complications that may require more specialized care. A team approach, which includes the primary cardiologist, adult congenital heart disease specialist, and maternal–fetal medicine, is proving to improve the care of such complex patients.