Coronary chronic total occlusion (CTO), defined as complete occlusion of a coronary artery for at least 3 months with thrombolysis in myocardial infarction (TIMI) grade 0 flow, represents one of the most complex situations in the field of interventional cardiology.1 Despite an incidence of CTO in patients with stable coronary artery disease (CAD) undergoing coronary angiography of 18–46%, the number of percutaneous coronary interventions (PCIs) for CTO is still less than 4% of all percutaneous revascularizations.2–4
In fact, the high rate of complications and failures of CTO revascularization, together with the lack of supporting data from randomized clinical trials, discourage interventional cardiologists from embracing this procedure. However, thanks to the progress of technology and to greater operator experience, the percentage of successful revascularizations has increased over the years, reaching approximately 90% in specialized centers.5 In addition, the number of procedural complications and adverse clinical events has also decreased significantly.6 Although randomized trials conducted so far have failed to demonstrate superiority of CTO revascularization strategies over medical therapy alone in terms of hard endpoints, observational studies and meta-analysis suggest a wide range of benefits of interventional treatment.7–9
Anatomy of Chronic Total Occlusion
In the context of CTO treatment, the assessment of risks and benefits of the intervention and the planning of the procedure are essential to achieve a successful revascularization. The first step is to carefully analyze the anatomy of the occlusion and the presence of collateral circulation. To do this, it is necessary to obtain accurate coronary angiography. Several authors suggest the use of simultaneous double coronary injection every time contralateral collateral circulation is present. This consists of injecting the contrast at the level of the donor vessel, followed 2–3 seconds later by injection into the CTO target vessel. During dual injection angiographic film exposure should be prolonged and the image acquired at low magnification avoiding panning, in order to gain a precise definition of both the lesion and collateral vessels.10
CTO consists of an atherosclerotic plaque and a thrombotic component that can be homogeneous or made up of several layers of differently organized tissues as a result of multiple thrombotic episodes occurring at different times.11 The proximal endoluminal part of the plaque (proximal cap) can be more or less hard depending on the age of the occlusion. Usually older lesions have a calcific cap that is more difficult to cross. Conversely, more recent occlusions are characterized by a softer cap that is easier to cross. The proximal cap is morphologically classified in three ways: blunt, tapered, and ambiguous. A tapered cap is usually less resistant to penetration than a blunt cap, making it crossable with low penetration force guidewires. In the case of proximal cap ambiguity, defined as the inability to accurately define the subsequent course of the vessel, it may be necessary to use a selective contrast injection with microcatheter, intravascular ultrasound (IVUS), or coronary CT angiography (CCTA) in order to better define the cap anatomy.12,13
To correctly define the complexity of the lesion, other characteristics such as length, tortuosity and composition of the occlusion should be defined. Long (>20 mm), tortuous, and calcified chronic occlusions make lesion crossing more difficult. They are also associated with a higher complication rate. Finally, the presence, size, and course of collateral vessels are fundamental to the planning of a retrograde approach, which may be the strategy of choice in the case of ambiguous lesions or antegrade technique failure.
Rationale for Revascularization of Chronic Total Occlusion
Symptoms and Quality of Life
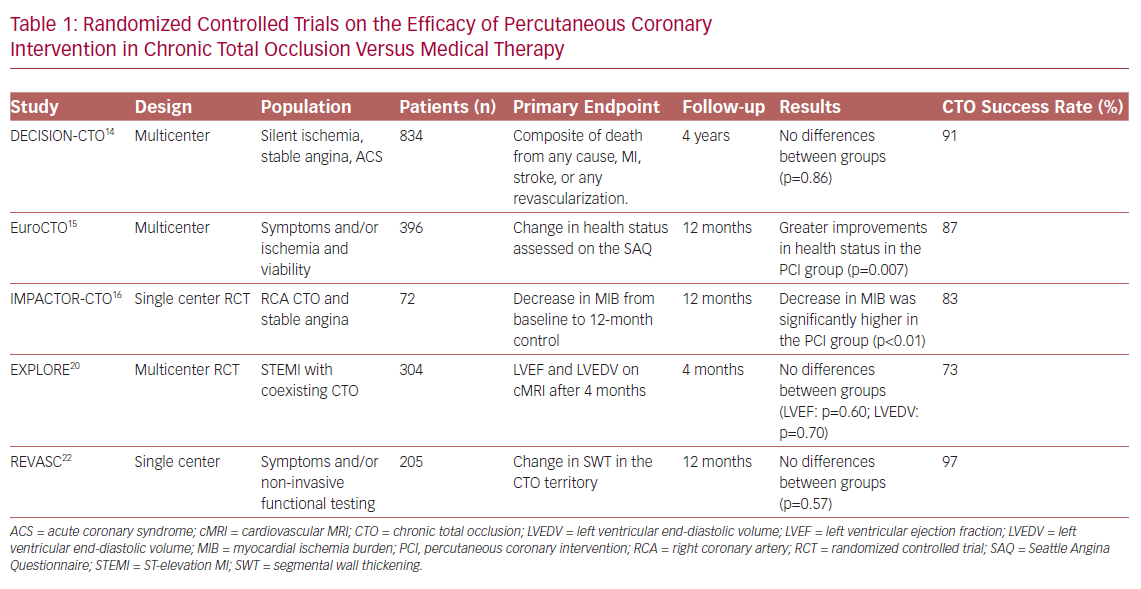
It is widely established that CTO PCI carries advantages in terms of improving symptoms compared with drug therapy alone. Looking at randomized trials in which angina and quality of life (QoL) were taken as endpoints, only the Drug-Eluting stent Implantation versus optimal Medical Treatment in patients with ChronIc Total OccluSION (DECISION CTO) study failed to demonstrate superiority of PCI compared with pharmacological treatment.14 In contrast, the Randomized Multicentre Trial to Evaluate the Utilization of Revascularization or Optimal Medical Therapy for the Treatment of Chronic Total Coronary Occlusions (EuroCTO study) and the Impact on Inducible Myocardial Ischemia of PercutAneous Coronary InTervention versus Optimal Medical TheRapy in Patients with Right Coronary Artery Chronic Total Occlusion (IMPACTOR CTO study) showed, at 1-year follow-up, advantages of percutaneous revascularization in reducing angina and improving QoL, assessed with the Seattle Angina Questionnaire (SAQ; Table 1).15,16
These differences could be due to the limitations found in the DECISION CTO trial, such as the slow and early termination of enrollment, the high percentage of cross-over in both arms, the high frequency of PCI for non-CTO lesions and the inclusion of patients with mild or absent symptoms.14 Moreover, the study was not focused specifically on CTO-only lesions: many patients with multivessel disease and one CTO were randomized to PCI of all vessels besides CTO, versus PCI of everything including CTO (thus the real impact of CTO on symptoms was not assessed). Finally, in the first days after randomization there was a cross-over rate of approximately 20% from the conservative to the PCI treatment, which may also have affected the final results.14
A recent study conducted by Hirai et al. analyzed 1,000 consecutive patients with high-grade refractory angina undergoing CTO PCI and showed that successful revascularization leads to higher improvement in the SAQ Angina Frequency and SAQ Summary Scores compared with unsuccessful PCI (35.0 ± 26.8 versus 18.8 ± 28.9, p<0.01; and 34.2 ± 19.4 versus 22.5 ± 20.8, p<0.01, respectively).17 This suggests that patients who may receive greater benefit from CTO revascularization are those with the highest degree of ischemia.
Further confirmation of the efficacy of percutaneous revascularization in terms of improvement of symptoms was obtained from a meta-analysis conducted by Joyal et al., with a lower persistence of angina in the group of patients who underwent successful revascularization.18 A better QoL, due to improvement of the depression-related symptoms common in patients with CAD, was also seen.19 The presence of refractory angina can lead to psychological distress and a depressive state. From this point of view, revascularization of CTO could lead to an improvement in health status in patients with angina and depression, as reported by Yeh et al.19
Regional and Global Left Ventricular Dysfunction
The improvement of left ventricular (LV) systolic function in patients undergoing CTO PCI is one of the most explored outcomes. In the randomized controlled multicenter trial Evaluating Xience and Left Ventricular Function in Percutaneous Coronary Intervention on Occlusions After ST-Elevation Myocardial Infarction (EXPLORE), 304 patients with ST-elevation MI (STEMI) and coexisting CTO in a non-infarct-related vessel were enrolled.20 The aim of the study was to evaluate, using MRI, the improvement of LV function and the reduction of LV end-diastolic volume in patients treated with PCI compared with those on medical therapy alone. The study showed, no advantages of PCI compared with drug treatment at 4 months after the acute event. However, the slow enrollment (leading to potential selection bias) and the relatively low PCI success rate (73%) may have affected the results. Moreover, the evaluation of the outcome only 4 months after randomization could have represented a further limitation. And, as evidenced by Bondarenko et al., myocardial recovery in many cases may be delayed.21
Similarly to the EXPLORE trial, the Recovery of Left Ventricular Function After Stent Implantation in Chronic Total Occlusion of Coronary Arteries (REVASC) trial at 1 year showed no differences between the two groups (CTO PCI versus medical therapy) in terms of changes in segmental wall thickening (SWT) in the CTO territory.22 However, the result could have been influenced by the revascularization of non-CTO stenosis in the group treated with medical therapy. Also, PCI for non-CTO lesions may have increased the flow in the collateral circulation, leading to recovery of areas of hibernating myocardium even in the group of patients without CTO PCI. This speculation arose from subgroup analysis that showed, in patients with less complex CAD (SYNTAX score <13), an improvement in SWT only in patients undergoing CTO revascularization. A further limitation of the study was the lack of assessment of myocardial viability on MRI. SWT was shown to improve significantly only in segments with <75% transmural infarct, with a significant remaining viable myocardium.23 Finally, in patients included in the study, mean LV ejection fraction (LVEF) was normal.22 A recent meta-analysis of 34 studies with a total of 2,804 patients, by Megaly et al., showed that successful revascularization of a CTO is associated with a significant increase in LVEF, especially in those with lower baseline values.24
Arrhythmic Events and Sudden Cardiac Death
Chronic total occlusion of an infarct-related coronary artery has been associated with higher risk of ventricular arrhythmia or appropriate ICD shock.25 Ventricular arrhythmias in patients with previous infarction arise in the myocardial area surrounding the fibrous scar.26 At this level, in patients with CTO, hypoperfusion could represent an arrhythmic substrate and favor the occurrence of ventricular tachycardia. Therefore, CTO revascularization, by restoring blood flow in the area close to the fibrotic scar, may generate positive electrical remodeling and reduce arrhythmias. A recent meta-analysis that assessed ventricular arrhythmias in patients with CTO has shown that CTO is associated with an increased risk of ventricular arrhythmia and all-cause mortality.27 Therefore, it is reasonable to consider defibrillator implantation in patients with an infarct-related CTO.
Mortality and Major Adverse Cardiac Events
There is a great discordance in the literature between observational studies and randomized clinical trials, regarding hard endpoints such as mortality and major adverse cardiac events (MACE). In the DECISION CTO trial, which assessed all-cause mortality, MI, revascularization, stroke and MACE, no advantages were found in the PCI group compared with medical treatment alone. One of the weaknesses of the study, in addition to those already mentioned above, was the exclusion of patients with LVEF <30% who appear to benefit more from revascularization.28 In contrast, data from several registries showed an increase in survival in patients undergoing successful recanalization compared with those with unsuccessful PCI.29,30
A meta-analysis of 25 studies (28,486 patients) conducted by Christakopoulos et al. showed that compared with failed procedures, successful CTO PCIs were associated with a lower incidence of death, stroke, coronary artery bypass grafting (CABG) and less recurrent angina.31 Furthermore, another more recent meta-analysis suggested possible advantages of revascularization of CTOs, compared with medical therapy, in terms of all-cause mortality, cardiac death and MACE.7 Finally, the REVASC study evaluated MACE, defined as total mortality, MI and any clinically driven repeat revascularization, at 12 months as a secondary endpoint. Although the trial was not powered for clinical endpoints, the CTO PCI group had a lower risk of MACE than the group with medical therapy alone.22
Recommendations for Revascularization of Chronic Total Occlusions
Current European and US recommendations for percutaneous revascularization of CTOs are derived from the randomized clinical trials conducted so far. The European guidelines recommend CTO PCI (Class IIa, level of evidence B), in patients with expected reduction of ischemia in a tributary territory and/or angina relief.32 At variance with US guidelines, CTO revascularization should be performed by an expert operator in patients with appropriate clinical indication and suitable anatomy.33
Risk Scores
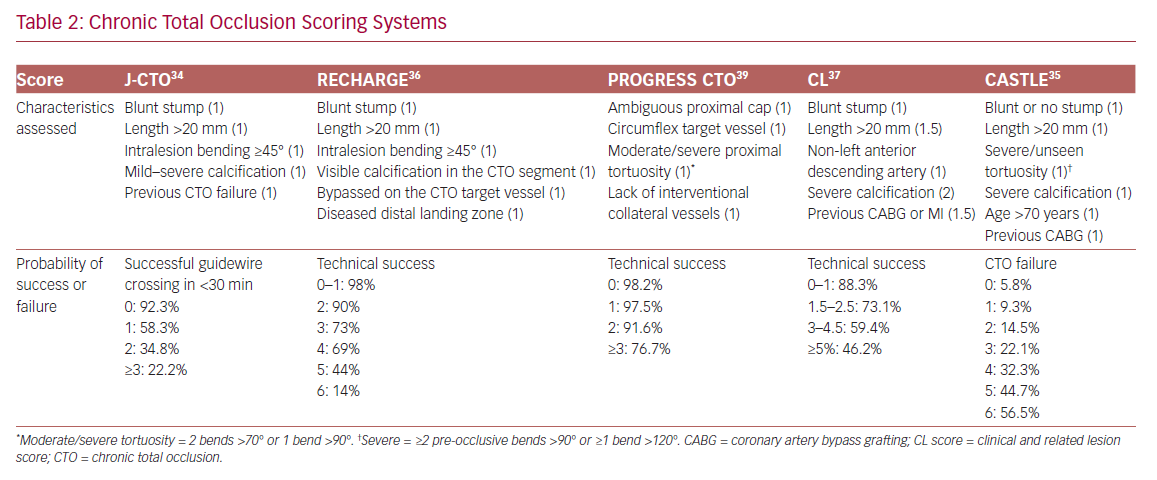
In order to define the degree of lesion complexity and predict the technical success of CTO, several scores have been proposed (Table 2). The Japan-CTO (Multicenter CTO Registry of Japan) score (J-CTO score) was developed to estimate the likelihood of successful guidewire passage of the CTO body within 30 minutes.34 The score consists of five variables, and 1 point is assigned to each: previous CTO failure; blunt stump of proximal cap; mild–severe calcification; intralesion bending ≥45°; and occlusion length >20 mm. Patients are classified into four grades of difficulty: easy (J-CTO score 0); intermediate (J-CTO score 1); difficult (J-CTO score 2); and very difficult (J-CTO score ≥3).34 In the EuroCTO (CASTLE) score the variables considered are: CABG history; age (70 years); stump anatomy (blunt or invisible); tortuosity; length of occlusion >20 mm; and extent of calcification.35 Tortuosity is defined as severe when the CTO vessel contains either two or more pre-occlusive bends >90° or at least one bend >120°; and moderate when it contains two bends >70° or one bend >90°. The CTO is defined as straight if the pre-occlusive segment contains a bend <70°.
Other popular scores are the Prospective Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS CTO), the Registry of CrossBoss and Hybrid Procedures in France, the Netherlands, Belgium and United Kingdom (RECHARGE), and the clinical and lesion-related (CL) scores, which have been widely validated, as demonstrated in a meta-analysis by Karatasakis et al.36–39
Strategies to Cross a Chronic Total Occlusion
There are four strategies to cross a CTO: two antegrade and two retrograde. In both approaches, the lesion can be crossed by passing through the true lumen of the vessel or through the subintimal space (using dissection and re-entry techniques). They are therefore defined as follows: antegrade wire escalation (AWE), antegrade dissection and re-entry (ADR), retrograde wire escalation (RWE), and retrograde dissection and re-entry (RDR).
Antegrade Approach
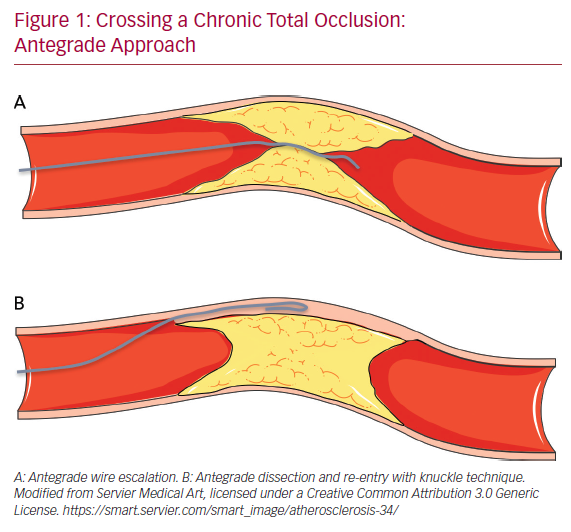
The antegrade wire (or AWE) technique consists of advancing various guidewires in an antegrade direction to cross the CTO while remaining within the true lumen of the vessel (true to true). Usually, for proximal cap penetration, tapered polymer guidewires with a low-penetration power soft-tip (Fielder family guidewires; Ashai Intecc), allowing advancement along visible or invisible microchannels, are the preferred first choice. If the operator encounters difficulties in crossing the lesion, they can switch to guidewires with greater penetration force. In general, when the proximal cap is blunt, guidewires with intermediate penetration force (Gaia 2nd–3rd [Ashai Intecc] or Pilot 200 [Abbott Vascular]) are used. If ineffective, guidewires with high penetration force (Conquest Pro 9-12 [Ashai Intecc] or Hornet 10-14 [Boston Scientific]) are used. In addition, the use of a microcatheter, placed near the lesion, may be helpful to increase the support and the penetration power of the guidewire (Figure 1).
Once the proximal cap has been penetrated, de-escalation to less penetrating guidewires can be carried out (quick and precise changing of guidewires is made easier by the presence of a microcatheter, because the path already run by the guidewire followed by a microcatheter does not need to be run again by the new guidewire, which can instead be used immediately at the level where the previous guidewire stopped). There are several methods for advancing the guidewire in the true-to-true lumen technique. In the sliding technique, using tapered polymer guidewires with soft tips, slight rotational movements and cautious advances are used to cross the lesion. The drilling technique, performed when there are harder caps, consists of advancing the guidewire, making rotational movements less than 90°. In the presence of more calcified lesions the operator may proceed with the penetration technique in which stiff guidewires are pushed without rotational movements.
Finally, in the push and torque technique the operator advances and rotates the guidewire until the hardest portion of the plaque is reached and then redirects it to a softer part. Once the lesion is crossed, in order to understand where the guidewire is located, it is necessary to perform the contralateral injection with the acquisition of at least two orthogonal projections. If during the wiring escalation technique, the guidewire accidentally reaches the subintimal space, it is possible to proceed with the dissection and re-entry technique. Alternatively, the guidewire can be left in the subintimal space as a reference, and a second guidewire advanced through the plaque (parallel guidewire technique).40 The first dissection and re-entry technique to be described was the subintimal tracking and re-entry (STAR) with a knuckled guidewire advanced in the subintimal space (Figure 1B).
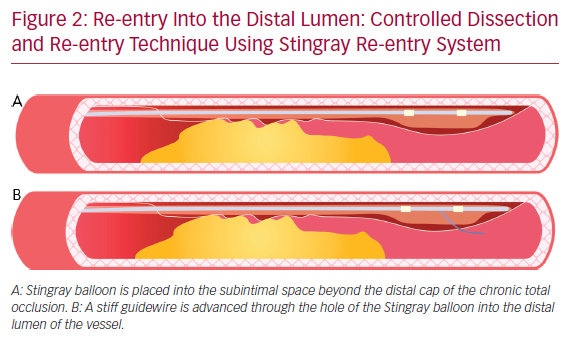
The subsequent re-entry into the distal lumen was unpredictable and uncontrollable.41 This implies that the stent is implanted in a long subintimal tract with higher risk of branch occlusion and vessel restenosis. A more recent technique with a particular flat balloon characterized by the presence of two juxtaposed holes (Stingray; Boston scientific) has been developed, allowing a controlled re-entry and collateral preservation. Once the balloon has reached the subintimal space beyond the CTO segment, a very stiff guidewire (or the dedicated Stingray guidewire [Boston Scientific] or a Hornet 14 [Boston Scientific] or a Confianza PRO 12 [Ashai Intecc]) is advanced through the hole in the luminal side, to regain the lumen of the distal vessel (Figure 2). A recent study conducted by Karatasakis et al. showed that limited dissection and re-entry techniques can lead to a lower risk of restenosis and target vessel revascularization.42
Retrograde Approach
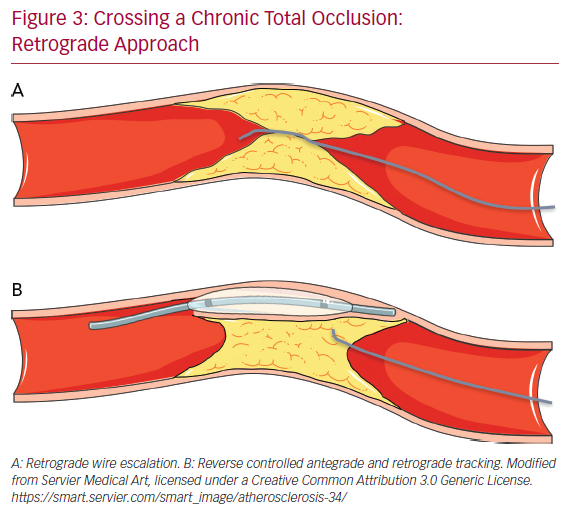
The retrograde approach is mainly used in complex anatomical settings or when antegrade methods have failed. The introduction of the retrograde approach has led to a successful rate of revascularization of approximately 90%.43 The first step in the retrograde approach is to pass the collaterals. There are several types of collaterals depending on the anatomy of the coronary tree and the specific occlusion: epicardial or septal, contralateral or ipsilateral. Moreover, in post-bypass patients the bypass itself can be used as a conduit to tackle the CTO using a retrograde approach. The careful assessment of collaterals is fundamental to the choice of the most appropriate collateral to cross. The passage of guidewires and microcatheters through the collateral channels is not without risk. One of the most frequent complications of CTO revascularization is coronary perforation, which may occur also during attempts to cross collaterals: specifically, epicardial collaterals are at higher risk for this type of complication due to their anatomical position close to the pericardium.
Conversely, the risk of perforation is lower (but still present) in the case of crossing septal collaterals. Once the collaterals are passed with guidewire (usually soft dedicated guidewires such as Fielder XT-R [Ashai Intecc], Sion [Ashai Intecc], Suoh03 [Ashai Intecc]) and with microcatheter, the next step is to pass in a retrograde fashion the CTO body, from the distal cap to the proximal cap. It is important to note that usually the distal CTO cap is anatomopathologically softer than the proximal cap because it is exposed to lower arterial pressures. This helps for an easier passage of guidewires through the distal CTO cap. In the RWE technique, as well in the AWE technique, guidewires with higher penetration power are progressively inserted into the distal cap (Figure 3A). In the case of failure of the true lumen to true lumen retrograde approach, or when a long lesion is present, it is necessary to use a dissection and re-entry technique such as the reverse controlled antegrade and retrograde tracking (CART; Figure 3B). With this technique the subintimal space is reached with an antegrade guidewire that is subsequently expanded with a balloon in order to permit the advancement of the retrograde guidewire from the subintimal space back to the true lumen.44
Chronic Total Occlusion Equipment
A deep knowledge of guidewire and microcatheter properties is crucial for any attempt at CTO revascularization.
Guidewires
To better understand the peculiarities of each guidewire, it is necessary to analyze every single component. Guidewires are composed of four elements, as follows.
The first element is the core: it is the inner part of the guidewire, which is usually composed of stainless steel, nitinol or both (dual core). In general, a stainless core provides excellent support, pushability (amount of force needed to advance the guidewire once it has penetrated a lesion) and torqueability (ability of the guidewire to transmit efficiently a rotation from the proximal to the distal end). Conversely, the nitinol core has excellent flexibility and trackability (ability of the guidewire to follow the tip around bends and tortuous vessels). Beyond the composition of the core, its thickness is directly related to the support of the guidewire.
The second element is the tip: this is the distal part of the guidewire and has two different designs depending on the extension of the core to the tip. The core-to-tip design consists of a direct connection between the two parts, allowing great torqueability and good tactile feedback. In contrast, when the core is shorter and does not reach the tip a small metal ribbon bridges the gap, ensuring flexibility and good shape retention.
The tip is also responsible for the penetration power (penetrability), defined as the force needed to bend the tip against resistance. Penetration power is directly related to tip stiffness and inversely related to the tip area. Therefore, tapered guidewires have a greater penetration power compared with guidewires with a non-tapered tip. Guidewires with high penetration force are used for crossing calcified lesions when softer guidewires have failed.
The third element is the body: this is the part of the guidewire that surrounds the core. It may be composed entirely of spring coils or a combination of spring coils and polymers (plastic). Polymer-jacketed guidewires provide advanced slip performance and good deliverability.
Fourth, the coating: the guidewires can be coated with hydrophobic or hydrophilic material. Hydrophobic coating (silicone based) gives the advantage of greater tactile feedback and a lower risk of unintentionally ending up in the subintimal space. Hydrophilic-coated guidewires are more slippery and are suitable to cross microchannels, tortuous vessels and small collaterals. Sometimes guidewires are coated with hydrophilic material with the exception of the tip, which is hydrophobic, providing at the same time slipperiness and optimal tactile feedback.45
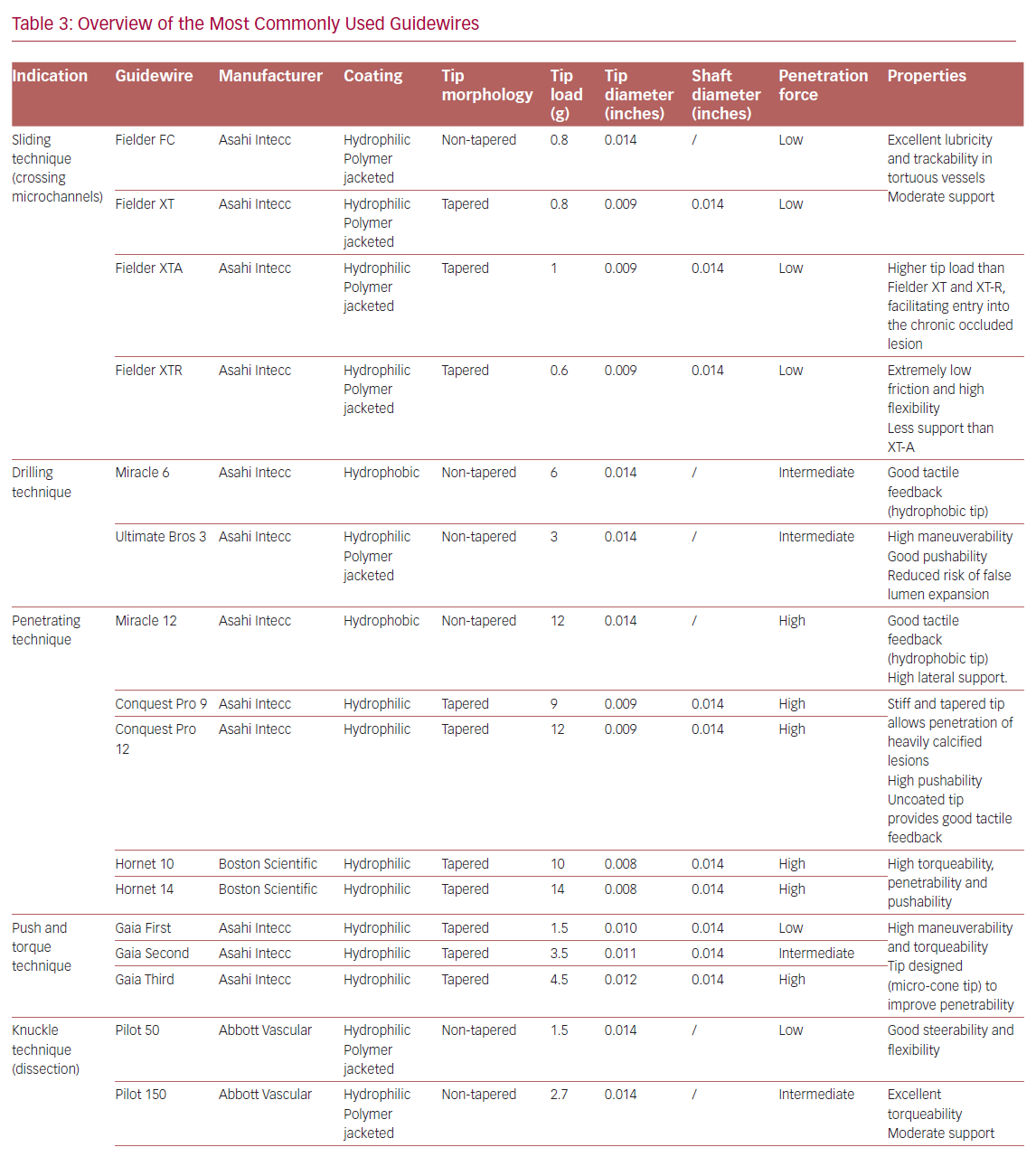
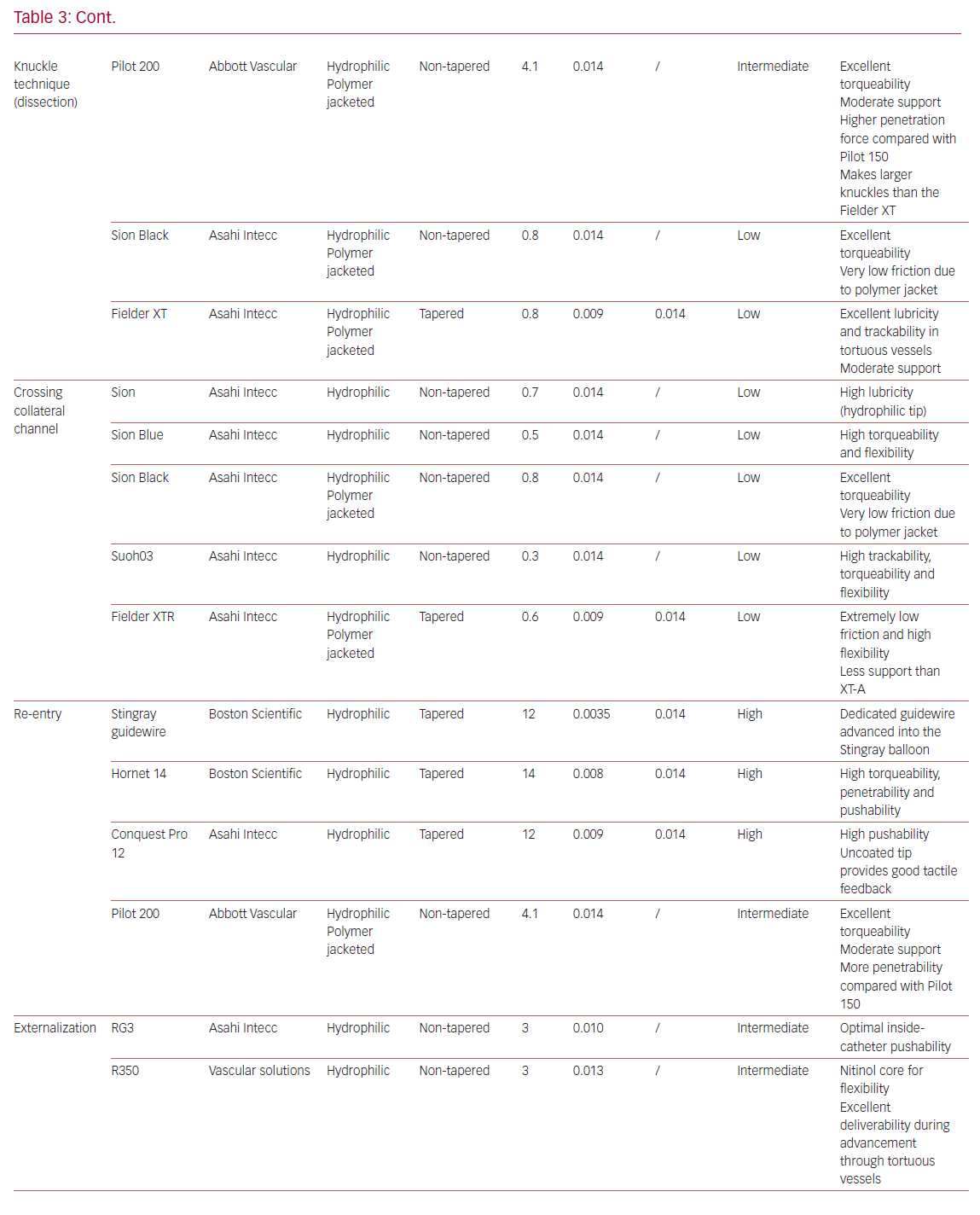
Table 3 summarizes the main properties and indications of the guidewires most used in the treatment of CTOs.
Microcatheters
Microcatheters are an essential tool in the treatment of CTOs. Basically, these are used in order to increase guidewire support and penetration power, to allow easy guidewire exchange and to perform selective contrast injection. Microcatheters have several features that differentiate them from each other, making them specific for precise situations. In general, large-diameter microcatheters are used to increase guidewire tip stiffness during the AWE technique. Conversely, small microcatheters are more useful in the case of tortuosity. Dual lumen microcatheters are used to perform the parallel guidewire technique and wire escalation when the side branch originates near the proximal cap, to wire the side branch of a bifurcation and to gain the main distal vessel when the guidewire achieves the side branch located near the distal cap. In the latter case, the pullback performed in order to redirect the guidewire from the side branch to the main vessel could lead to loss of the path already gained.46
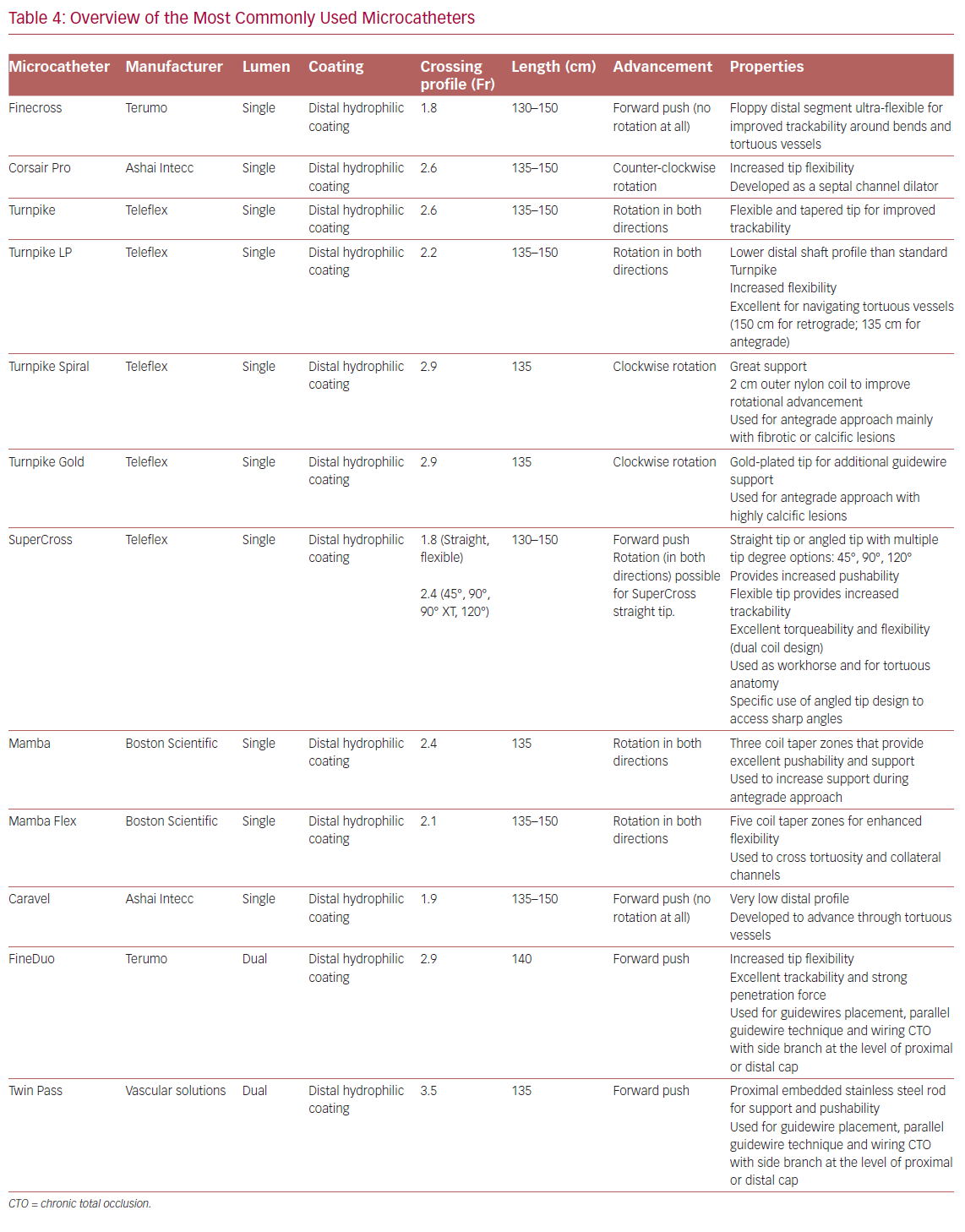
Table 4 summarizes the main properties of and indications for the microcatheters most used in the treatment of CTOs.
Role of Intravascular Ultrasound in Chronic Total Occlusion
In the treatment of CTOs, IVUS may be a useful tool to deal with some complex scenarios. One of its main uses is in the case of proximal cap ambiguity. In this condition, if a good-sized collateral branch originates near the lesion, the IVUS catheter can be inserted in the proximal segment of the side branch, thereby visualizing the exact location of the CTO cap. This allows correct puncture of the proximal cap, reducing the risk of inadvertent advancement of the guidewire into the subintimal space or even outside the vessel structure. Sometimes during AWE techniques, the guidewire can accidentally end up in the subintimal space, creating a large dissection and leading to collapse of the distal true lumen. For this reason, the distal part of the vessel cannot be seen on simple angiography, making the following part of the procedure dangerous. In this situation, the IVUS catheter may help to identify the distal true lumen and allow safe and successful re-entry. The operator, after pre-dilation of the subintimal space with a small balloon (1.5 mm), advances the IVUS catheter into the false lumen, identifying the point of re-entry proximal to the distal cap. Then, under the guidance of IVUS, a second guidewire with high penetration support is advanced to gain the true lumen. IVUS can be particularly useful to tackle ostial lesions with the RDR technique. In this situation the IVUS catheter is placed proximal to the occluded ostium, at the level of the bifurcation, guiding the retrograde passage of the guidewire from the subintimal space to the proximal true lumen. Finally, IVUS is widely used for stenting optimization. Although the randomized studies conducted so far have not shown clear advantages of IVUS-guided revascularization compared with angiography alone, the use of this technique could reduce the risk of complications, especially in selected complex settings.47,48
Algorithm for Crossing Chronic Total Occlusions
Although the antegrade approach is generally preferred because it is associated with a lower risk of complications, the retrograde technique is necessary to achieve successful PCI in many procedures. Sometimes retrograde techniques may represent the first strategy in the presence of a complex anatomy or ambiguity of the proximal cap. Several algorithms have been proposed to simplify the revascularization and to choose the best approach. The most frequently used are the hybrid approach and the Asia Pacific algorithm.5,49
The hybrid approach consists of positioning two guide catheters in the right and left coronary arteries for complete assessment of the CTO, by doing simultaneous double injection with two guiding catheters. The main criteria to assess the CTO are: type of proximal CTO cap (well defined or ambiguous), length of the CTO segment (less or more than 20 mm), presence of collaterals that can be used for retrograde procedures (so called ‘interventional collaterals’), and quality of the distal vessel after the CTO (diseased distal vessel, distal CTO cap at bifurcation). According to all possible combinations of these four parameters, one of the four techniques (AWE, ADR, RWE or RDR) is chosen as the first strategy. Having the set up with double guiding catheters allows the switch from one technique to another during the procedure in the case of failure of the previous one. Thus the CTO is tackled from every perspective possible. Several registries have highlighted the importance of the hybrid algorithm, which has a high PCI success rate (86–91%) and a relatively low percentage of procedural complications (1.7–2.6%).50,51
The Asia Pacific algorithm maintains some common features with the hybrid algorithm such as the evaluation of the proximal cap, the quality of the distal vessel and the presence of collaterals. In contrast, the length of the occlusive stenosis is not considered. Furthermore, it is recommended to use the CrossBoss catheter (Boston Scientific) in the presence of intrastent occlusive restenosis, the Stingray system (Boston Scientific) in the case of failure of the antegrade approach, and the parallel guidewire technique and IVUS-guided wiring as a bail-out strategy in the antegrade arm.
Complications of Chronic Total Occlusion Percutaneous Coronary Intervention
Recent advances in the field of CTO have increased the rate of successful revascularization and reduced periprocedural complications. A report from the National Cardiovascular Data Registry showed that the incidence of MACE in CTO percutaneous procedures, although decreasing over time, is still higher compared with non CTO PCI.4 Furthermore, the development of procedural complications has also been associated with a worse long-term health status that has further discouraged interventional cardiologists from actively dealing with CTO percutaneous revascularization. Registry data show a total complication rate ranging from 2.6% to 9.7%.52–54 Several complications may occur during CTO PCI, including coronary perforation, periprocedural MI, arrhythmias, cardiogenic shock, stroke, major bleeding, donor vessel thrombosis, contrast-induced nephropathy, radiation damage and death.53 These have been independently associated with a retrograde approach, advanced age and lesion complexity defined according to the J-CTO score.
One of the most frequent and feared complications of CTO PCI is coronary perforation.55 This usually occurs as a result of collateral channel damage during a retrograde crossing attempt or due to an accidental exit of the guidewire during wire escalation (both antegrade and retrograde) using high penetration force guidewires. Perforation can occur at the level of epicardial vessels, with an increased risk of cardiac tamponade, or at the level of septal collaterals, and is usually self-limited and does not require specific treatment. In the presence of coronary perforation that is not self-limited, it is crucial to stop the bleeding promptly.
The first step should involve the placement of a balloon close to the perforation. In the majority of cases this may be enough to resolve the complication and prevent cardiac tamponade. However, if it is not sufficient, it becomes necessary to implement additional strategies such as positioning a covered stent or coils or injecting autologous fat, thrombin or microbeads at the level of the perforation.56 Of note, the injection of these substances should never be performed in the case of connection between the perforation and the left chamber, because of the high risk of embolization and stroke. Pericardiocentesis is needed in cases of cardiac tamponade. However, in most cases coronary perforations do not cause hemodynamically significant pericardial effusion.57 Finally, cardiac surgery may be required as a bailout option for percutaneously unresolvable bleeding.
Another complication due to coronary perforation is the intramural hematoma, also called ‘dry tamponade’.58 Vessel perforation may occasionally lead to an important blood extravasation within the myocardial wall or in localized structures next to the heart itself (typically in post-CABG patients in whom the pericardium has several adhesions), causing ventricular or atrial compression and stiffening and subsequent risk of cardiogenic shock. The treatment of this complication is complex: the first step is to stop the bleeding; second, the blood extravasation should be decompressed, but these localized blood collections may be difficult to reach percutaneously. Sometimes only supportive measures to generate increased intracardiac pressure (such as hydration) in order to compensate for the external pressure, are sufficient to bridge the patient to a stable situation that then allows the hematoma to reabsorb by itself. In the case of development and persistence of hemodynamic compromise, ventricular assist device could be used to stabilize the patient as a bridge to recovery.
Periprocedural infarction still represents one of the feared complications of CTO PCI. This can occur as a consequence of collateral branch occlusion (in particular during uncontrolled dissection and re-entry technique) or in the case of thrombotic occlusion of the donor vessel. The former may be avoided through the use of more controlled re-entry systems, the latter by means of excellent anticoagulation with an activated clotting time >300–350 seconds. Finally, of the most frequent extra-cardiac periprocedural complications, those related to vascular access are notable. In recent years, attention has been paid to the type of access, with the aim of minimizing the use of femoral access and of large introducer sheaths (8 Fr).24
Importance of Access and the Minimalistic Hybrid Algorithm
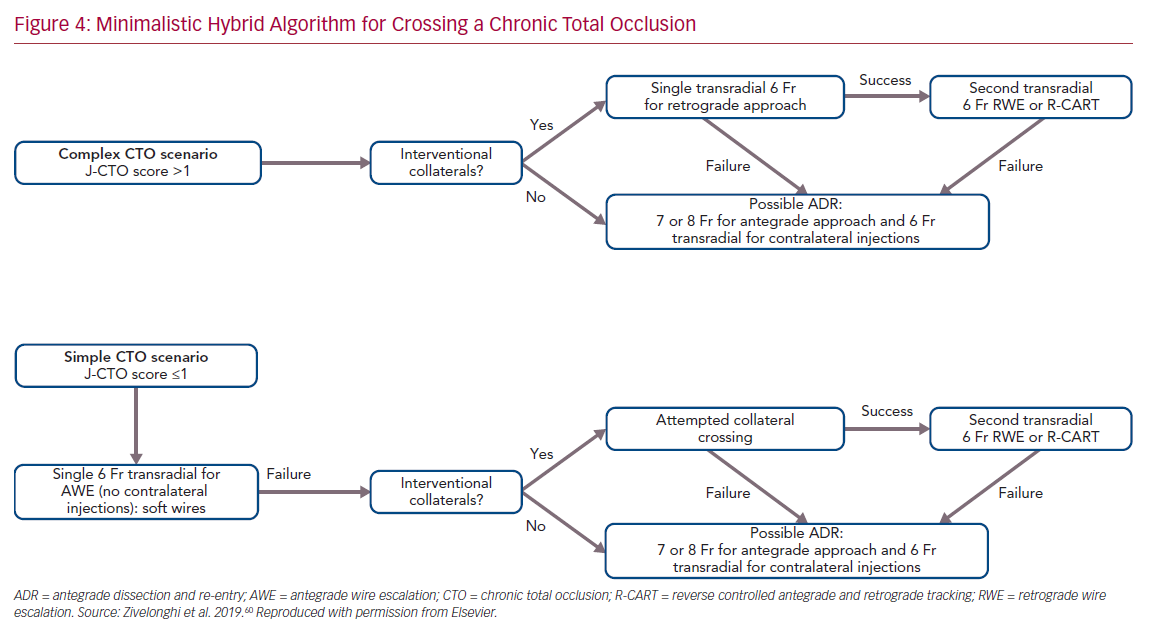
The choice of arterial access plays an important role in the procedure. The type of access depends on operator preference and on the anatomical characteristics of the CTO. In general, complex lesions, such as those with a very resistant proximal cap, require large guide catheters (7 or 8 Fr) for greater passive support. Consequently, femoral access is usually preferred. In contrast, radial access, which is now increasingly used for CTO PCI, is associated with similar procedural success but with a lower risk of major bleeding compared with femoral access.59 We recently proposed an approach based on the use of all four possible hybrid strategies to cross the CTO, but with the aim of reducing the rate of femoral approach, double access and periprocedural complications.60 This approach, called the minimalistic hybrid algorithm, has shown promising results, ensuring a high revascularization success rate using the radial approach in 90% of cases and a single catheter approach in nearly 60% of procedures (Figure 4).61
Conclusion
CTO treatment represents one of the most challenging and complex procedures in the field of interventional cardiology. International guidelines based on the results of the available randomized controlled trials (RCTs), support CTO revascularization only in selected cases. It is a generally accepted opinion that in patients with refractory angina, CTO PCI improves QoL. Its effect on ventricular function and other hard endpoints such as mortality and MACE, however, is still debated; although RCTs and observational studies have consistently suggested the possible advantages of CTO PCI when compared with optimal medical treatment in some subgroups of patients, for instance in those with depressed EF and/or evidence of myocardial hibernation. Therefore, despite improvement in technique and greater operator skill, CTO PCI remains a complex procedure with a non-negligible incidence of complications. For this reason, accurate selection of patients who may benefit from this procedure is needed, and specific algorithms leading to a systematic approach to this type of procedure, are welcome.