Pregnancy is a physiologic challenge, with significant hormonal, metabolic, and hemodynamic changes. Cardiac output is objectively increased by the fifth week after the last menstrual period and continues to grow by approximately 45 % by 24 weeks in the normal, singleton pregnancy. This is facilitated by elevations in heart rate and stroke volume and a decrease in systemic vascular resistance. These changes return to the prepregnant state approximately 2 weeks after delivery.1 The hemodynamic demands of these adaptations and accompanying hormonal fluctuations can create a “stress” situation, which may uncover underlying metabolic abnormalities or cardiovascular disease (CVD), the leading cause of maternal mortality in pregnancy.2 Fortunately, CVD in pregnancy is rare, however, it requires clinicians from multiple specialties to coordinate efforts to care for two patients at once.
The hormonal and metabolic changes of normal pregnancy are intertwined, with insulin resistance, hypercoagulability, and immunologic dysfunction each playing important roles in fetal development while potentially contributing as risk factors for cardiovascular ischemia. Although the exact mechanisms of pregnancy-associated insulin resistance are complex, normal human pregnancy is associated initially with adipose accretion, a progressive 50 % decrease in insulin-mediated glucose disposal, and a subsequent 200–250 % increase in insulin signaling to maintain euglycemia.3,4 Pregnancy is a known prothrombotic state, with the rate of venous thromboembolism (VTE) approximately four to five times that of a nonpregnant female.5 Additionally, major shifts occur in the maternal immunologic status with the downregulation of pro-inflammatory cytokines and acceptance of the fetus and his or her immunological differences.6
The prevalence of myocardial ischemia is low overall in pregnant women, with a variable presentation from asymptomatic to cardiogenic shock or sudden cardiac arrest.7,8 The incidence and prevalence of myocardial infarction and ischemia related to pregnancy is expected to increase more women are delaying child-bearing to later years, with a technical definition of an age >35 years as advanced maternal age (AMA).9–11 Although obstructive atherosclerotic heart disease occurs in reproductive age women, the pathophysiology of ischemic heart disease (IHD) in women also includes a greater proportion of nonobstructive coronary disease than found in men.11 However, patients with stable angina and normal coronary arteries or non-obstructive plaque burden have been shown to have increased risks for major adverse cardiovascular events (MACE).12,13 Pregnant patients may present with a medical attention with a clinical context of acute coronary syndrome (ACS) with chest discomfort, dyspnea, or other more atypical symptoms such as referred pain, nausea, or profound fatigue. Electrocardiography (ECG) and assessment of cardiac biomarkers are essential to diagnosis, per practice guidelines. The absence of ST-elevation on an ECG would likely suggest non-ST-elevation ACS (NSTEACS). NSTEACS can by further subdivided as a non-ST-elevation myocardial infarction (NSTEMI) with elevated myocardial injury biomarkers, or unstable angina (UA).14
The CVD complication rate during pregnancy is 0.2–4.0 % in Western societies, with hypertension occurring in 6–8 % of all pregnancies. Management of these complications is not standardized and is largely guided by Level of Evidence (LOE) C data, that is, consensus opinions of experts, case reports, and adaptations of care standards.15,16 Of those with CVD during pregnancy, adult congenital heart disease comprises 75–80 % in Europe and North America, while rheumatic valvular heart disease comprises the 56–89 % of pregnancy-associated CVD in non- Western countries.17,18 The occurrence of an acute MI has a rate of one in 35,700 pregnancies with a high mortality rate of 7.3 %.19 Pregnancy increases the risk for MI three to four times over the nonpregnancy state, which could be due in part to the physiologic “stress test” of increased cardiac output.20 The presentation of a contemporary cohort of pregnant patients with IHD was predominantly in the third trimester or postpartum period and 95 % had chest pain. Etiologies of IHD are variable between different retrospective cohorts, but the major etiologies are primarily coronary dissection (35–56 %), atherosclerosis (35 %), thrombus (22–35 %), and “normal” coronary anatomy (11 %).21,22
Diagnostic Evaluation
There are few data on the management of patients with pregnancy associated MI (PAMI) and ACS. Pregnant patients presenting with ischemic symptoms should be evaluated at the emergency department with the same attention paid to history, biomarkers, and ECGs, according to the most recent guidelines.23 Additional imaging may be warranted in patients with atypical symptoms or in whom initial testing is not diagnostic. While multiple imaging modalities are available to evaluate cardiac function, nonionizing methods, especially echocardiography, are generally preferred and provide ancillary diagnostic information including myocardial and valvular function. Often, it can be difficult to delineate common symptoms of pregnancy, such as mild shortness of breath or edema, from ischemia or heart failure. A transthoracic echocardiogram should be considered in a woman who has dyspnea out of proportion to her baseline. Ionizing imaging studies in the acute setting, such as nuclear imaging, CT, and coronary angiography should be selected when it is determined that the potential information gained outweighs potential maternal and fetal risk. While cardiac MRI does not involve ionizing radiation, there are few indications for use in the acute setting. MRI has not been shown to have a harmful effect on the fetus, with teratogenesis, miscarriage, or acoustic damage, however, a strong magnetic field and substantial noise of greater than 100 dB, although attenuated by the mother’s body, are of potential concern.24–26 Gadolinium-based contrast has been shown to induce teratogenic effects in animal models in doses above any clinical indication, however, case series studies have not shown an association between gadolinium contrast and adverse fetal outcomes.27,28 The American College of Radiology (ACR) recommends that a risk–benefit analysis be performed before MRI, and that gadolinium-based agents may be given to a patient under a consensus agreement by the referring physician and radiologist when no other imaging modalities are favorable, and when the imaging cannot be performed after delivery.29,30
Concerns about exposing the mother and fetus to ionizing radiation are legitimate, as there is increased sensitivity of glandular breast tissue to ionizing effects leading to an increased risk for breast cancer, and there is no threshold radiation level below which radiation would be considered “safe” for a fetus.31 Radiation exposure is undesirable in the pregnant patient for the potential of direct and more likely scatter radiation to the developing fetus. As many precautions as possible, including minimizing low-dose fluoroscopy where appropriate, minimizing cine imaging, and perhaps shielding along the patient’s lower back if possible might mitigate fetal exposure.32 Due to the high incidence of coronary dissection in this population, special procedural precautions are recommended, including careful guide engagement of the coronary arteries and limiting the number and pressure settings of contrast injections.
Etiologies for Acute Ischemia in the Pregnant Patient
Coronary Artery Dissection
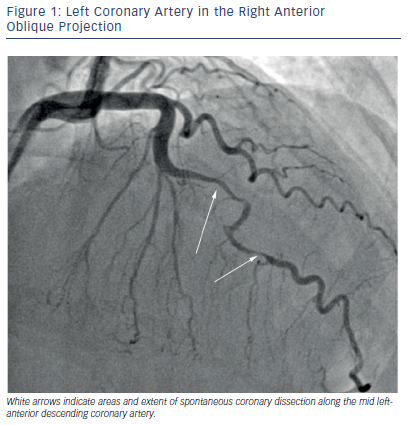
A review of 150 cases of PAMI revealed that the most common etiology was spontaneous coronary artery dissection (SCAD) (43 %), followed by atherosclerotic disease (27 %), and thrombus without atherosclerosis (17 %). Normal coronary anatomy was present in 11 %, however, the extent of coronary evaluation with intracoronary imaging modalities is likely underutilized.20 Patients with SCAD typically present with left anterior descending (LAD) artery involvement, and a significant proportion may present with multivessel involvement.33 SCAD is diagnosed angiographically, and emerging data suggest three different angiographic classifications of SCAD: Type 1 with multiple radiolucent lumen, Type 2 with diffuse stenosis, and an “atheroscleroticmimic”, Type 3.34 An angiogram of SCAD within the LAD artery, best classified as Type 2, is shown in Figure 1. Histology reveals that SCAD is a consequence of dissection within the coronary artery media or intima and intramural hematoma formation.35 SCAD may or may not angiographically demonstrate a double lumen, which is best appreciated by optical coherence tomography or intravascular ultrasound.36,37
The pathophysiological explanation behind SCAD is overall unknown, however, pregnancy induces a state of increased responsiveness to angiotensin II, catecholamines, and endothelial dysfunction.38 Alterations in the coagulation–fibrinolysis system is hypothesized to contribute to SCAD with a pregnancy-induced prothrombotic state.
Additionally, excess estrogen and progesterone promote changes in the arterial wall, which could contribute to medial breakdown.39 The significant increase in blood volume, cardiac output, and abrupt hemodynamic stresses in delivery and postpartum have also been hypothesized to contribute to an increased chance at dissection.40 Pregnancy is a risk factor for SCAD, with a majority of the cases occurring in the third trimester or post-partum period. Additionally, case reports reveal pregnancy-associated SCAD as more frequent in women >30 years of age and in multiparous women.20 Fibromuscular dysplasia (FMD) is associated with and may be a causal factor in SCAD, although the prevalence of FMD in pregnancy-associated SCAD is unknown.41–43
Thromboembolic
Thrombophilia in pregnancy is more often associated with VTE than arterial thromboembolism.44 Paradoxical embolus is an unusual cause of MI, and is more commonly associated with cryptogenic stroke.45,46 Literature on pregnancy and paradoxical embolus leading to cardiac ischemia remains limited to case reports. In two instances, the patients were documented to each have a patent foramen ovale (PFO) along with a Factor V Leiden mutation.47,48 Coronary thrombosis without atherosclerotic disease causing ischemia is rare, however, 45 % of women with pregnancies complicated by acute MIs were smokers.40 Cigarette smoking in pregnancy is associated with increased platelet activity, further contributing to a pro-coagulant cascade.49
Atherosclerosis
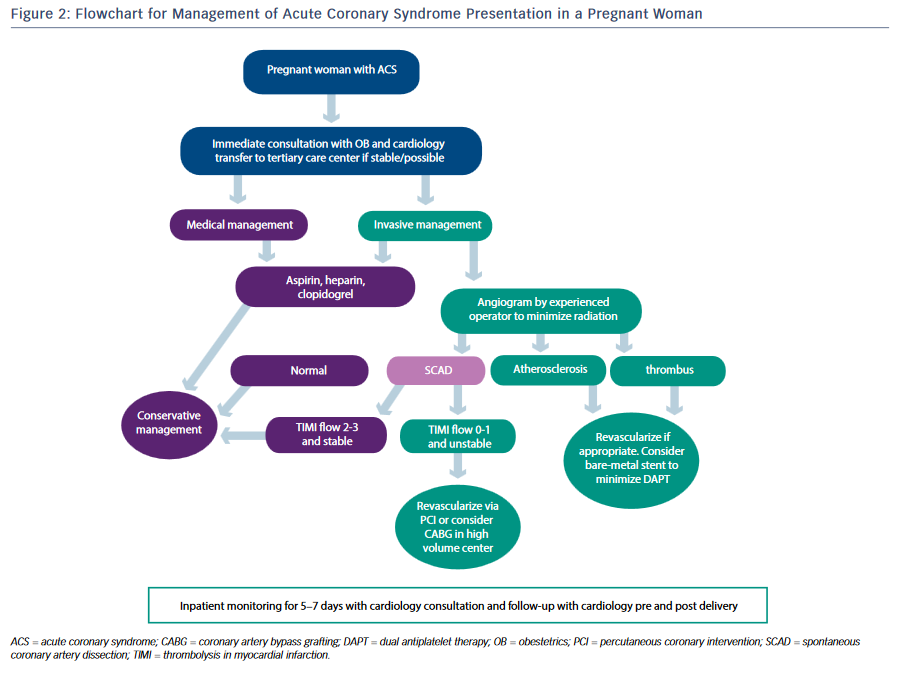
Atherosclerotic heart disease, or coronary artery disease (CAD), is responsible for the largest proportion of CVD among women and men, and for nearly one-third of all deaths worldwide.50 Age is strongly associated with CAD, and as more women delay childbearing to later years the number of ischemic events in pregnant women is also expected to increase.51,52 A recent study evaluated 43 women with prior MI or a history of ACS and 50 pregnancies, over a time span of 7 years and across six academic medical centers. Women with pre-existing CAD and prior MI events were at a higher risk for coronary ischemic events with pregnancy, found to comprise five patients with one death, three ACS/MI episodes, and one case of heart failure. Established risk factors were present in 80 % of those patients, and 60 % had a history of cigarette smoking.22 Figure 2 is an algorithm for the management of the pregnant patient with ACS.
Risk Factors for Pregnancy-associated Coronary Ischemia
It is difficult to ascribe risk factors to one etiology of coronary ischemia versus another due to low event rates. Additionally, since many studies are retrospective and typically from tertiary referral hospitals, many cases may be unknown in the greater community. It would appear that many clinical risk factors are shared between the two largest contributors of pregnancy-associated coronary ischemia: coronary dissection and atherosclerosis. A significant risk factor is age. Acute MI can occur in all stages of pregnancy, however, a retrospective review published in 2008 of 103 women with MI associated with pregnancy revealed that 72 % of patients were >30 years and 38 % were >35 years.40 Several years later another retrospective study of 150 women with PAMI revealed that 75 % were >30 years and 43 % were >35 years.20 The average age in ACS presentation due to SCAD was 42.6 years with an 82 % prevalence in women.53 Among women in the same cohort who had peripartum SCAD, the average age of presentation was 36 years. Smoking is a known risk factor for CAD and ischemic events in general, and it is similarly a commonly present risk factor among PAMI, varying from 25 % to 45 % within studies.20,40
Multiparity was reported to be present in 47–66 % of women with PAMI.20,40 Multiparity is known to cause increases in serum estradiol, progesterone, and other hormones of pregnancy.54,55 These two facts support the mechanism of increased doses of hormones as a direct link to abnormal endothelial function, thrombosis, and increased susceptibility to the known risk factors of atherosclerosis such as smoking, hyperlipidemia, and hypertension. Fertility treatments and hormone replacement therapy were present in two out of 15 patients with SCAD recurrence in one center.53
Preeclampsia is maternal hypertension, typically developing in the third trimester of pregnancy, characterized by proteinuria, and is progressive without treatment, possibly leading to liver and kidney failure and seizures (eclampsia).56 It is a leading cause of maternal and fetal morbidity and mortality; however, in the retrospective studies of acute MI and pregnancy, the prevalence of preeclampsia was not shown to be noticeably different than the age-adjusted rates, around 6 %.20,40,56,57 Preeclampsia is associated with future CVD, including IHD.58 A retrospective cohort of over one million women without CVD prior to first delivery were followed for cardiovascular outcomes in the Cardiovascular Health after Maternal Placental Syndromes (CHAMPS) study.59 Preeclampsia comprised 49 % of those with maternal placental syndrome, making it the most common disorder, followed by gestational hypertension (28 %) and placental abruption (15 %). The adjusted hazard ratio of preeclampsia for the development of premature CVD was 2.1 (95 % CI [1.8–2.4]).
The Nationwide Inpatient Sample for 2000–2002 was queried for pregnancy-related discharges. Out of the 859 discharges with a diagnosis of acute MI, multivariable logistic regression analysis revealed that the biggest comorbidities were hypertension (OR 21.7; 95 % CI [6.8–69.1]), thrombophilia (OR 25.6; 95 % CI [0.2–71.2]), and diabetes (OR 3.6; 95 % CI [1.5–8.3]).8 Transfusion for hemorrhage was also associated with MI, although it is difficult to ascribe the increased risk for MI to the transfusion of red blood cells or to agents, such as methylergonovine, administered to women with hemorrhage due to uterine atony, and known to induce coronary vasospasm.60 This elegant analysis also highlighted the compounded impact of smoking toward an increased risk for PAMI. Although female smokers are twice as likely as nonsmokers to suffer from MI, pregnant smokers have an eightfold risk for MI. This is similar to the risk for MI in female smokers taking oral contraceptives.61
Treatment for Ischemic Coronary Disease in Pregnancy
Medical Management
After the diagnosis has been made for STEMI or NSTEACS in the pregnant patient, expedited consultation with cardiologists who have expertise in the care of pregnant patients and high-risk obstetricians is recommended. The medical management has important differences, in efforts to minimize risks to the fetus. Medications have risk factor categorical classifications for use in pregnancy based in animal and human data when available, with progressively increased fetal risk from A to D and also a category X. Category A drugs show no risk or evidence of harm in controlled human studies. Category B drugs demonstrate no risk in animal studies, however, there are no controlled human trials. Category C drugs demonstrate either unavailable or insufficient data in animals or women, or possible fetal risk in animal studies, without controlled human studies. Category D drugs have evidence of potential fetal risk in animals, however, the benefits in pregnant women may be accepted despite the risk, such as life-threatening condition to the mother. Category X drugs are contraindicated in pregnant women due to demonstrated fetal abnormalities in animals or humans.62 Fibrinolytics do not cross the placenta, and case reports exist of successful thrombolysis in pregnant patients suffering an acute MI. However, the general opinion is of relative contraindication and extensive discussion among consultants if fibrinolytic therapy is remotely considered.63–65 Additionally, due to the high prevalence of SCAD and “normal” coronary anatomy in pregnant patients with ACS, the benefit of fibrinolytic therapy may not outweigh the risk.
Due to the low frequency of MI in pregnancy, anti-platelet, and anticoagulation therapies are managed similarly to nonpregnant patients, as pregnancy has never been evaluated in clinical trials of these agents. Aspirin is given a class D designation, mostly due to animal models exposed to high-doses resulting in premature fetal ductus arteriosus closing.66 Aspirin at a low dose (75–100 mg) is safe to use in pregnancy with no increased maternal or fetal bleeding risks or effects on the ductus arteriosus on meta-analysis. Additionally, aspirin was not found to increase the risk for bleeding from neuraxial anesthesia when continued through delivery.67 The platelet P2Y12 receptor blockers of clopidogrel and prasugrel are considered pregnancy class B agents, while ticagrelor is a class C agent with limited literature comprising one case report.68,69 Regarding anticoagulation, unfractionated heparin does not cross the placenta, but is a pregnancy class C agent. Due to the neurologic effects on the neonate of benzyl alcohol, which is present in some formulations of heparin as a preservative, some practices have limited the administration of heparin to pregnant patients to preservative-free heparin, if possible.70,71 Enoxaparin is a class B agent, does not cross the placenta, and is well tolerated in pregnancy from a bleeding perspective when stopped before a planned delivery. The direct thrombin inhibitors of bivalirudin and argatrobran are class B agents due to animal studies, and their use is limited to case reports of pregnant patients with heparin-induced thrombocytopenia.72,73 European guidelines on the management of pregnancy and heart disease recommend the use of clopidogrel and avoidance of glycoprotein IIb/IIIa inhibitors, prasugrel, ticagrelor, and bivalirudin.74 In a pregnant patient who had recently received a bare metal stent, clopidogrel was stopped 7 days prior to delivery. Eptifibatide was used as a bridging agent and discontinued 12 hours prior to neuraxial analgesia, with clopidogrel resumed within 24 hours.75
Invasive Management
Percutaneous coronary intervention (PCI) with stents remain the treatment of choice in an acute STEMI, although this does not take into account the high-prevalence of SCAD in pregnant patients.76 Consultation with a high-risk obstetrician regarding the feasibility and duration of dual anti-platelet therapy should ideally occur before angiography. Additional precautions due to the high incidence of coronary dissection are to perform careful guide engagement of the coronary arteries and to minimize injections, using low-pressure.
Treatment for SCAD has been a controversial topic, with no randomized controlled data on management. Retrospective studies of SCAD reveal that half of these patients with ACS present with a STEMI, and likely from guidelines advocating early and invasive management for STEMI and NSTEACs, many of these patients have been treated with PCI.53 Some studies report favorable outcomes for PCI management of SCAD, and others report a high failure rate of PCI and a better prognosis of conservative management. The largest single-center study of 189 patients with SCAD reported a PCI procedural failure rate of 53 %, and more vessel occlusion with revascularization than conservative management (44/95 versus 18/94, respectively). The conclusion from this study was that a conservative strategy with observation may be preferable in those clinically stable patients with TIMI grade 2–3 flow.77
There are no data with respect to outcomes on bare-metal versus drugeluting stents in the pregnant patient. Bare-metal stents have been used more frequently than drug-eluting devices in pregnant patients, to reduce the length of dual anti-platelet therapy and potential bleeding complications surrounding delivery.40,78 A high rate of iatrogenic coronary dissection with angiography and stenting in one study suggests that a conservative and noninvasive approach be maintained for most pregnant patients, and that revascularization attempts remain for those with severe and proximal obstruction or hemodynamic compromise.20
Mode of Delivery
Pregnant women who have experienced an MI or ACS prior to or during their pregnancy should be delivered in a tertiary care center if possible, with a coordinated team consisting of a high-risk obstetrician, cardiologist, and obstetric anesthesiologist. Unless the woman is in active ACS or suffering an MI during labor, vaginal is the preferred mode of delivery. A large, retrospective study of 1,262 deliveries in women with heart disease concluded that planned caesarean delivery was of no significant benefit, and that vaginal delivery was associated with later delivery and greater birth weight.79 European guidelines advocate for vaginal delivery, with an individualized plan according to the patient’s condition. Acute heart failure decompensation, Marfan’s syndrome with an aorta >45 mm, acute or chronic aortic dissection should be considered for caesarean delivery. Additionally, patients on oral anticoagulation should ultimately be switched to unfractionated heparin and optimally delivered in a controlled manner, so that the risk of fetal bleeding, particularly intracranial bleeding, from oral anticoagulants is reduced.74 Vaginal delivery is associated with less blood loss and a decreased infection risk compared with caesarean delivery.80 Lumbar neuraxial anesthesia is recommended for reducing pain-associated sympathetic activation and as an anesthetic should the patient require emergent delivery.
Long-term Management
All women with known heart disease or those who experience an MI or ACS and desiring pregnancy should be referred to a cardiologist ideally before stopping birth control. Pregnant women with known heart disease should be managed by a high-risk obstetrician, preferably at a tertiarycare center, with a consultant cardiologist. Women with PAMI should be followed by a cardiologist closely before and after delivery. Counseling with respect to future pregnancies after an ischemic event must be tailored with the severity of the ischemic event, coronary anatomy and the severity of atherosclerosis, the status of her right and left ventricular function, and the need for dual antiplatelet or anticoagulation therapy.
Contraindicated medications during pregnancy include angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and statins due to teratogenic effects.81 An open dialogue about intentions for breast-feeding needs to begin before hospital discharge and continue in the postpartum appointments between the cardiologist, obstetrician, and patient to discuss the risk to benefit ratio of cardiovascular medications to both the infant and mother. Unfortunately, ACE inhibitors, ARBs, and statins as a class are likely to be avoided with respect to breast-feeding, as the data are inadequate and controversial. Metoprolol is a Category C drug for pregnancy, but is likely safe during breast-feeding. Atenolol should be avoided in all pregnant and breast-feeding patients.82
Risk in Future Pregnancies
Due to the paucity of data regarding myocardial infarction in pregnancy, not much is known about the risk for future cardiovascular events in subsequent pregnancies. A case series of pregnancy after a diagnosis of SCAD was examined in a single institution. Eight women with prior SCAD became pregnant, and seven had no cardiovascular complications. However, one woman had recurrent SCAD of the left main coronary artery and underwent emergent coronary artery bypass grafting (CABG) and subsequently developed posttraumatic stress disorder.83 As such, future pregnancy is not advised after SCAD, even if the prior SCAD was not associated with pregnancy.
Pregnant women with pre-existing CAD or a history of MI/ACS are at increased risk for angina, MI, ventricular arrhythmias, or cardiac arrest during their pregnancies, comprising 10 % from one retrospective review. The highest rates of cardiac complications are seen in women with atherosclerotic disease. Interestingly, the risk for neonatal adverse events, such as preterm labor, intrauterine growth restriction, or low birth weight, is greater for women with known CAD (30 %) than with known valvular or congenital heart disease (18 %), and is substantially higher than the risk in women without heart disease (7 %).22 The Cardiac Disease in Pregnancy (CARPREG) investigators reported that predictors of complications in pregnancy are: an impaired systolic function, any prior cardiovascular event including stroke, left-heart obstruction (mitral or aortic stenosis), or New York Heart Association (NYHA) function class II or above.17 The ZAHARA (in Dutch, Zwangerschap bij vrouwen met een Aangeboren HARtAfwijking) investigators limited their risk score to pregnant women specifically with congenital heart disease.84 Neither of these risk scores evaluated women with histories of MI or ACS, likely due to the low prevalence of pregnant women with known CAD.
Conditions in pregnancy that predispose the mother to future cardiovascular events after pregnancy are similar to other risk factors in the general population. Women with hypertensive disorders of pregnancy became diagnosed with hypertension 7.7 years earlier than women without pregnancy complications and were at a significantly increased risk for CVD with a hazard ratio of 1.21 (95 % CI [1.10– 1.32]).85 Maternal obesity and preeclampsia predispose the mother to cardiovascular events later in life.85,86
Women with prior MI or known CAD should undergo cardiovascular evaluation as part of preconception planning. While there are no specific guidelines for this evaluation, it is reasonable to perform physical examination, ECG, echocardiography, and functional testing for inducible ischemia in most women. In addition to those with prior SCAD, women with significantly reduced left ventricular function or heart failure or ischemic symptoms should be advised against pregnancy.
Conclusion
Although IHD is rare in pregnancy, the maternal mortality from heart disease is several fold above the nonpregnant population. The strongest risk factors for IHD in pregnancy are age, smoking, multiparity, and a history of MI or ACS. In this rare population, SCAD should remain high on the differential for PAMI, followed by atherosclerosis. Critical to the management of pregnant patients with IHD is a multidisciplinary and involved team of at least an obstetrician, noninvasive and interventional cardiologists, and obstetric anesthesiologists at a tertiary center. Additional cardiovascular follow-up, mitigation of risk factors, and counseling regarding plans for future pregnancy are imperative toward best possible outcomes.