Cardiac pacing has evolved considerably over the years from its initial introduction as a lifesaving measure by asynchronous ventricular pacing. Pitfalls of ventricular-only pacing were recognized relatively early, which led to the introduction of atrioventricular (AV) synchronous pacing that was subjected to rigorous clinical studies.1 A meta-analysis of major pacing mode trials revealed that AV synchronous pacing, either through atrial pacing (AAI) or dual-chamber pacing (DDD), compared with ventricular pacing (VVI) reduces the incidence of AF and stroke, but not heart failure hospitalization or mortality, even though some individual studies have shown a significant benefit of physiological pacing with respect to overall mortality and heart failure.1 However, persistent right ventricular apical (RVA) pacing can be associated with ventricular dyssynchrony and left ventricular adverse remodeling.2,3 Results of the Mode Selection Trial (MOST) showed that >40 % of DDD and >80 % of VVI were associated with increased risk of heart failure hospitalizations, and that risk of AF increased linearly with cumulative percentage ventricular pacing.2 Results of the Dual-Chamber and VVI Implantable Defibrillator (DAVID) trial showed that >40 % of VVI is independently predictive of heart failure hospitalization and death.3
In an effort to reduce the percentage of RV pacing, pacemaker programming algorithms such as Managed Ventricular Pacing (MVP) and Search AV+ (SAV+) (Medtronic), Reverse Mode Switch (RYTHMIQ, Boston Scientific), SafeR mode (LivaNova), Ventricular Intrinsic Preference (VIP, St. Jude Medical), and AV hysteresis and VP suppression (Biotronik) have been successful to some extent.4 However, these programming algorithms are of limited value in patients who are dependent on ventricular pacing. Although implanting an AAI pacemaker in patients with preserved AV, interventricular, and intraventricular conduction might seem appropriate, there remains a potential risk of developing AV block in the future.5,6 Pacing from RV sites other than RV apex have been evaluated in many studies. Systematic reviews and meta-analyses of trials comparing non-RVA sites to RVA pacing site have shown that non-RVA pacing may offer modest, but significant, hemodynamic benefit over RVA pacing.7,8 However, data regarding any advantage in exercise capacity, functional class, quality of life, or survival were limited and inconclusive.8 In patients with ventricular pacing indication and/or anticipated high-percentage ventricular pacing, and left ventricular ejection fraction (LVEF) >35–50 %9 or any LVEF,10 two major studies assessed usefulness of addition of an LV pacing lead to provide cardiac resynchronization therapy (CRT; or biventricular pacing ) compared with pacing without CRT (standard RV pacing). The Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK-HF) trial showed that implanting a biventricular pacemaker compared with RV pacing significantly improved the primary endpoint, which was a composite of death, urgent care visit for heart failure, or a 15 % increase in LV end systolic volume index (LVESVI), but this was driven mainly by increases in LVESVI.9 The Biventricular Pacing for Atrio-ventricular Block to Prevent Cardiac Desynchronization (BIOPACE) study revealed no superiority of biventricular pacing compared with RV pacing.10 Recently, His bundle pacing (HBP) has emerged as a novel physiologic strategy for achieving ventricular pacing, and has brought renewed hope and excitement into the pacing arena.
Although HBP was first reported by Scherlag et al. in 1967,11 the first significant clinical series in humans was not published until 2000 by Deshmukh et al.12 Despite that, HBP did not receive much attention until more recently and is now emerging as a promising modality to preserve synchronous ventricular pacing. Many studies have shown that HBP is feasible and safe, and offers better outcomes with respect to left ventricular end diastolic and end systolic diameters and systolic function, shorter interventricular electromechanical delay, improvement in quality of life, reduction in New York Heart Association (NYHA) class, and improvement in 6-min walk, when compared with RV pacing,12–24 and is effective for CRT.25–30 The objective of this review is to give a systematic appraisal of the history, feasibility, safety, techniques, efficacy, benefits, complications, and challenges, and to offer a future perspective, of HBP, as the search for the optimal pacing site continues.
History of His Bundle Pacing
The history of HBP has recently been reviewed in detail.31 The study of cardiac conduction system has accomplished several anatomical, physiological, pathophysiological, and therapeutic clinical milestones. The Czech anatomist and physiologist Jan Evangelista Purkinje was the first to describe, in 1839, the fibrous tissue (Purkinje fibers) that conducts stimuli along the ventricular endocardium to all parts of the heart. In 1893, Wilhelm His Jr, a Swiss cardiologist and anatomist, discovered the auriculoventricular bundle (His bundle) linking the atria to the ventricular septum, leading to the concept of His-Purkinje system. He pioneered studies in cardiac conduction and coined the term ‘heart block.’32 In 1899, Wenckebach described the phenomenon that later bore his name.33 In 1906, Dr Tawara, a Japanese pathologist, discovered the AV node and in his publication described a treelike structure of the AV node, the His bundle, bundle branches, and Purkinje fibers, which served as a pathway of AV conduction and excitation of mammalian heart.34 In 1907, in England Arthur Keith and Martin Flack discovered the sinoatrial node as the origin of cardiac pulsations.35 In 1924, Woldemar Mobitz, a Russian-born German physician classified second-degree AV block.36 In 1958, Alanis et al. demonstrated the first His bundle recordings using isolated perfused hearts of dog and cats.37
In 1967, Scherlag et al. were the first to describe His bundle recording and stimulation using the epimyocardial approach in dogs undergoing open chest surgery, and a year later were able to demonstrate direct HBP using an endocardial approach in dogs, followed by publication in 1969 of their technique for recording His bundle electrograms in humans.38 In 1970, Narula et al. demonstrated HBP in patients who underwent right heart catheterization, during which the pacing impulse to ventricular activation time was the same as the HV interval during normal sinus rhythm and remained constant at different pacing rates.39 In 1976 Williams et al. published results of their experiments in anesthetized open-chest dogs and defined non-selective HBP (capture His bundle and ventricle or atrium) versus selective HBP (capture of His bundle alone).40 In 1992, Karpawich et al. described a new endocardial electrode implant approach to permanent HBP in dogs during thoracotomy using a custom-designed active-fixation lead with an exposed helix.41
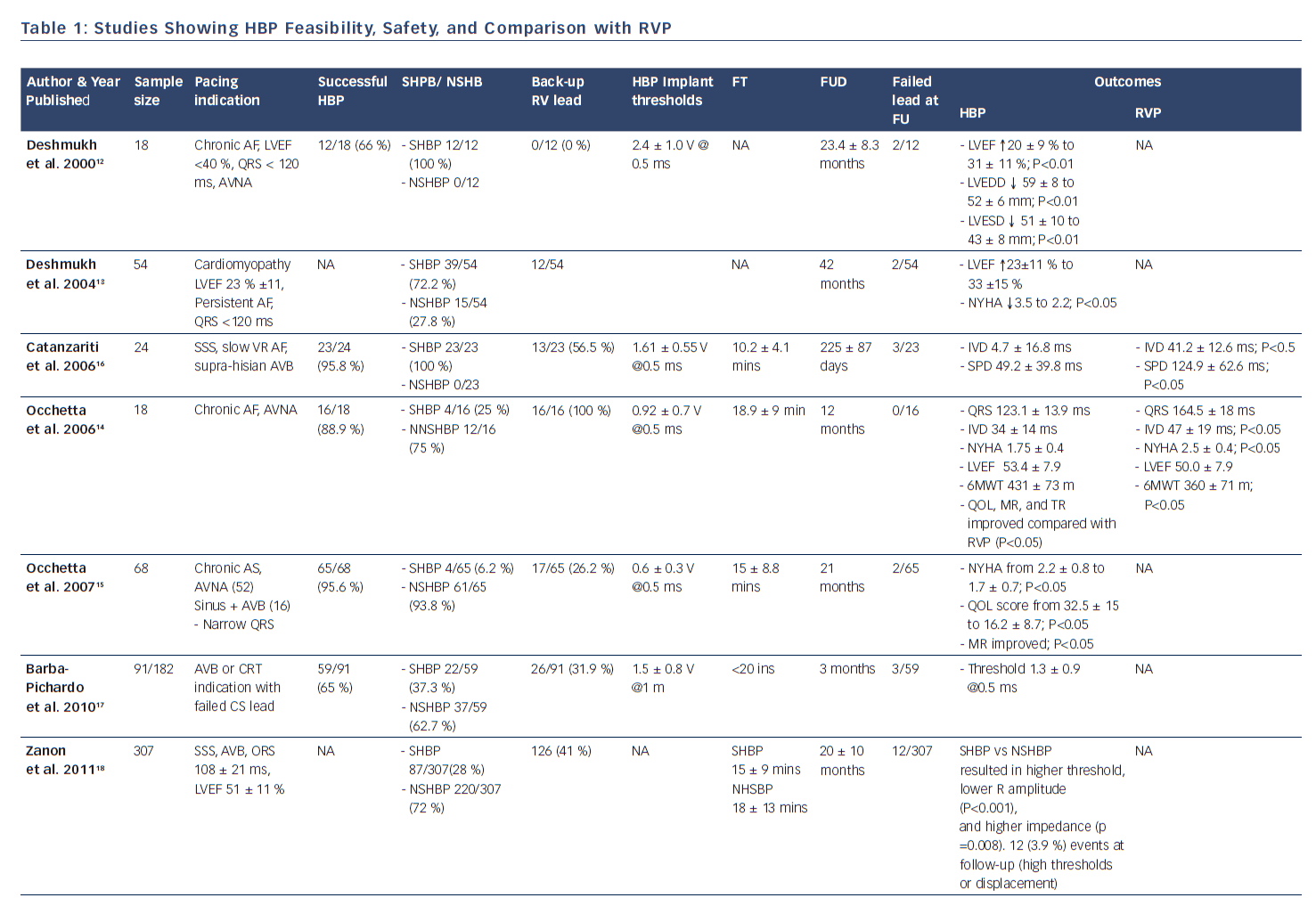
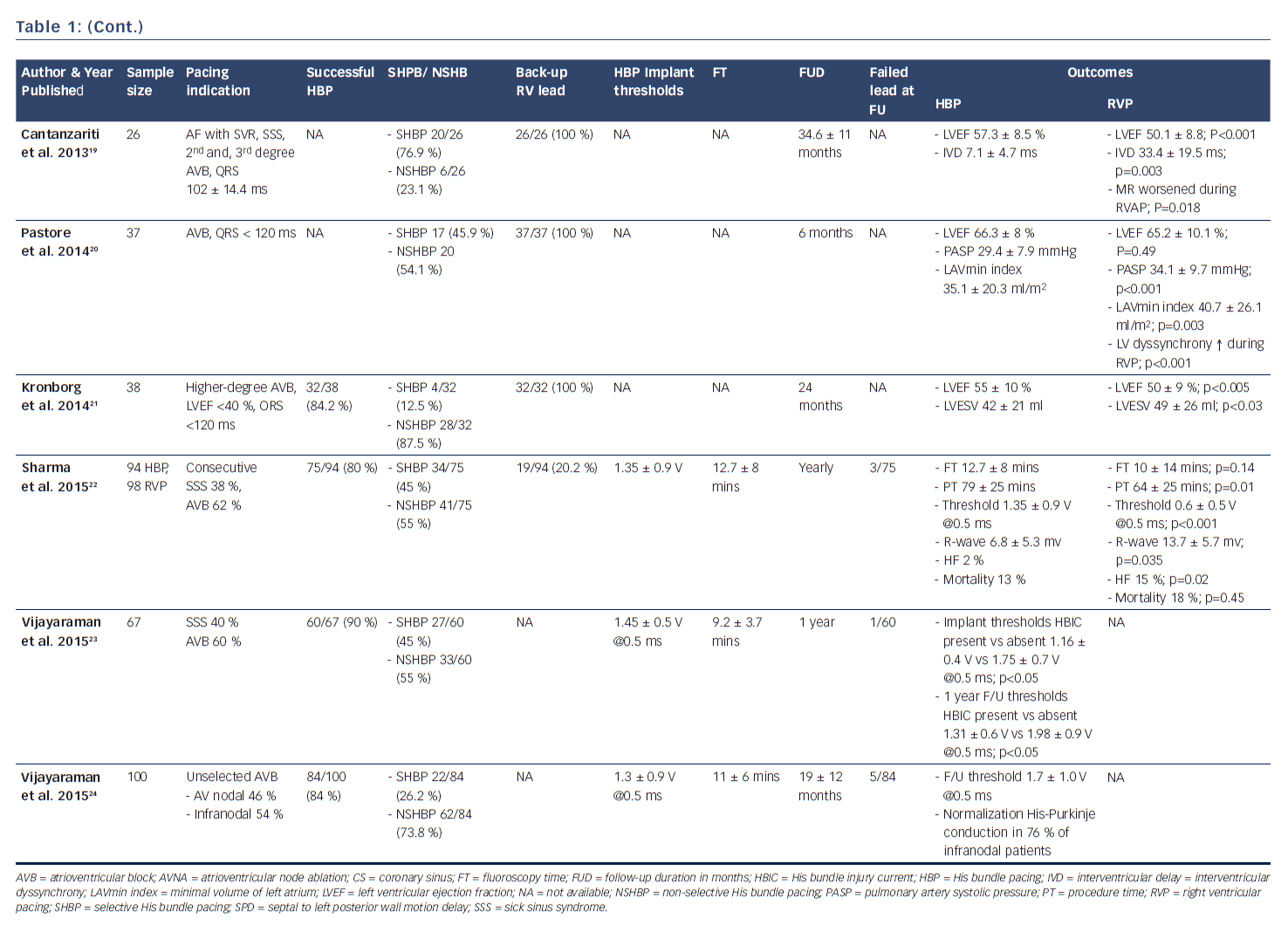
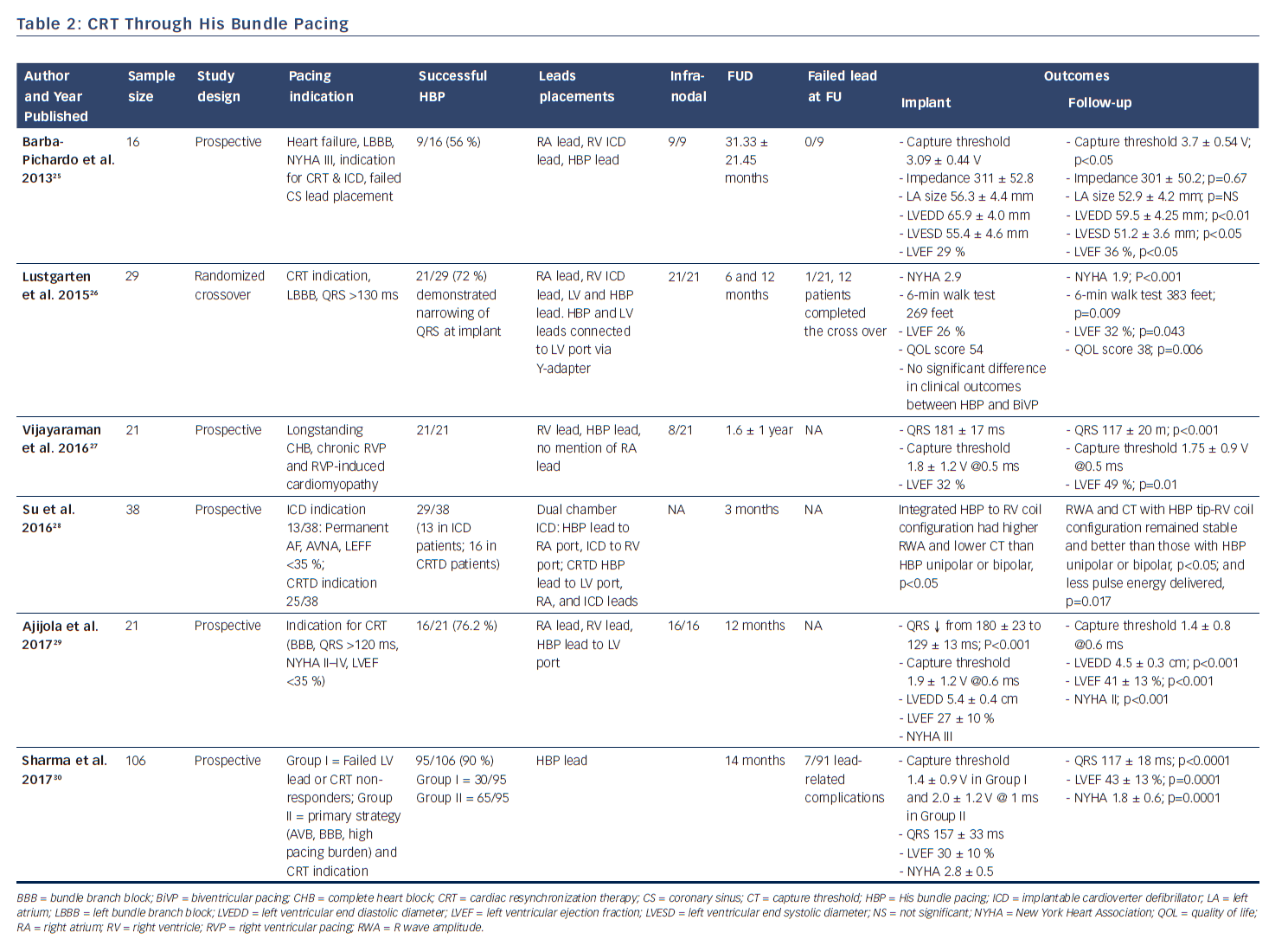
In 2000, Deshmukh et al. demonstrated that permanent direct HBP was feasible in 12 out of 18 patients with permanent AF and dilated cardiomyopathy undergoing AV node ablation, and at follow-up HBP resulted in a reduction of left ventricular dimensions and improved cardiac function.12 This landmark report ignited heightened interest in this area and further clinical studies have now successfully demonstrated the feasibility and safety of HBP and its impact on improved positive ventricular remodeling.13,15,17,23 These studies have shown the superiority of HBP compared with RV pacing with respect to ventricular synchrony and positive remodeling.14,16,19–22 More recently, safety in patients with infranodal AV block has been demonstrated (see Table 1).24 There is now accruing evidence that selective HBP provides cardiac resynchronization in CRT non-responders, in those who experienced failure of LV lead placement via the coronary sinus,25,30 and in all-comers with CRT indication (see Table 2).26–30
Feasibilty and Safety of His Bundle Pacing
After pioneering the publication of the first therapeutic clinical series of HBP in humans in 2000, Deshmukh et al. published results in 2004 showing that selective HBP led to significant improvements in NYHA class and left ventricular systolic function in 54 patients with severe cardiomyopathy, permanent AF, and narrow QRS after an average of 42 months of follow-up.13 In 2006, Zanon et al. conducted a feasibility study showing that direct HBP could be accomplished with a new system consisting of a steerable catheter and an active-fixation lead in 92 % of the patients in whom it was attempted.42 Since then, several publications across the world have corroborated these findings, as shown in Table 1. Most recently, data from high-volume HBP centers at Indiana University School of Medicine, Indianapolis, Indiana, and Geisinger Wyoming Valley Hospital, Wilkes-Barre, Pennsylvania, have demonstrated the feasibility, safety, as well as the reliability of the procedural technique using custom-made delivery sheaths and active-fixation leads.22–24,30,43,44
As shown in Table 1, the implant success rates in attempted HBP from studies that published the denominator number from which successful HBP was achieved varies from 56–95 %, with most recent studies showing improvement in implant success rates of as high as 70–90 % with enhanced implanter experience. The indications of pacing varied from sick sinus syndrome, permanent AF patients undergoing AV node ablation, AV nodal level of atrioventricular block with narrow QRS, and even infranodal block. HBP was performed with and without back-up RVP across studies. Some studies have demonstrated safety of HBP with long-term follow-up data in patients with infranodal block without back-up RV lead.24
It has been observed that pacing thresholds, sensing, and impedance parameters after HBP are reliably stable at follow-up.13,15,19,44 Although thresholds have been noted to be modestly higher during generator change than during implant, they remained relatively stable and reliable. Sensing and impedance parameters have also remained stable at follow-up. One study demonstrated that in 20 patients undergoing generator change at mean duration of 70 ± 24 months after initial implant, His bundle capture threshold at implant was 1.95 ± 1.1 V @ 0.5 ms. At the time of generator change, the HBP threshold was higher at 2.5 ± 1.2 V @ 0.5 ms (p=0.02). There were no significant differences in the sensing amplitude or pacing impedance at the time of generator change.44 Most importantly, the QRS duration in patients with narrow QRS at implant tended to remain unchanged with HBP at follow-up.15
Procedural Technique
Most published series have used the Medtronic implanting equipment systems for direct HBP,42,43 which includes delivery sheath and active-fixation lead utilizing a fixed non-extendable screw. There are isolated reports of successful deployment of traditional pacemaker leads with a retractable screw (Tendril 1488 or 1788, St. Jude Medical).17,25 The commonly used equipment necessary for HBP include an appropriately sized peel-away sheath inserted through the cephalic or axillary or subclavian vein, through which a steerable or deflectable (SelectSite, model C304 Medtronic Inc.) or non-deflectable (model C315) His bundle delivery catheter sheath is inserted. Through the chosen delivery sheath, a bipolar, non-stylet-driven, fixed-screw pacing lead (SelectSecure model 3830, Medtronic Inc.) is maneuvered to the His area. Some studies report the use of diagnostic multipolar catheters (such as CRD-2, St. Jude Medical) placed from the femoral or axillary veins to map a discrete local His-bundle electrogram with fluoroscopic guidance, before placing the His permanent pacing lead.29 However, most reported series have used the His bundle lead itself to directly map the His electrogram without need for a mapping catheter, during which unipolar His electrograms and His-ventricular and paced-ventricular intervals are measured by means of a multi-channel electrophysiology analysis systems (e.g. Cardiolab, Pruka GE) or directly on a Medtronic pacing system analyzer (model 2290) at sweep speeds of 50–100 mm/s. The lead is advanced in an antero-posterior (AP) fluoroscopic projection and placed with help of right anterior oblique (RAO) and left anterior oblique (LAO) projections. After identifying a His bundle electrogram by mapping the His bundle region, pacing is performed to confirm His bundle capture. The lead is then fixed into position by means of four to five clockwise rotations of the entire lead to anchor the non-extendable screw.42,43 The His bundle injury current is usually elusive at implant, with an experienced HBP center succeeding to register only 37 % success, while in the remaining 63 %, only the His bundle electrogram was recorded.23 When obtained, His bundle injury current is similar to myocardial injury current seen during RV pacing, and indicates direct contact of the fixation screw to the junctional tissue and is associated with significantly lower pacing thresholds compared with patients in whom the injury current was not recorded. Acute His bundle trauma can occur during manipulation and fixation attempts causing bundle branch block; however, complete resolution of conduction block usually occurs in majority of patients. Vijarayaman et al. observed trauma to the His bundle in 7.8 % of patients undergoing permanent HBP, with complete resolution of conduction block occurring in 68 % and persistence of right bundle branch block in 32 %.45
Selective Versus Non-selective His Bundle Pacing
Successful selective HBP (also referred to as direct or pure HBP) occurs when ventricular activation is occurring solely over the His–Purkinje system and is defined using the following criteria: (1) His–Purkinje-mediated cardiac activation and repolarization, as evidenced by ECG concordance of QRS and T-wave complexes; (2) the pacing spike-QRS interval being almost identical to the His–ventricular interval (this can be further confirmed by presence of late ventricular electrogram on the pacing lead, precluding the possibility of local capture); and (3) His bundle capture in an all-or-none fashion, as demonstrated by the absence of QRS widening at a lower pacing output.12,40,46
Non-selective HBP occurs when there is simultaneous capture of the His bundle and the basal ventricle and can be recognized from the surface ECG leads by changes in onset and amplitude of the QRS with appreciable T-wave alterations,40 and is defined by the following criteria: (1) no isoelectric interval between pacing stimulus and QRS; (2) recording His bundle electrogram on the pacing lead; (3) electrical axis of the paced QRS concordant with the electrical axis of the spontaneous QRS (if known); and (4) narrowing of QRS at higher output due to fusion between RV and His bundle capture and widening of QRS at lower output due to loss of His bundle capture, or vice versa.46,47 This was sometimes referred to as ‘para-Hisian pacing,’ but this term is no longer used in the context of permanent HBP to avoid confusion with para-Hisian pacing performed during electrophysiology studies to distinguish retrograde activation over AV node from that over the accessory pathway during intermittent His bundle capture.
As consistent and reliable capture of His bundle requires higher output than RV capture, selective HBP results in a higher pacing threshold than non-selective HBP.18 However, the lower pacing threshold in non-selective HBP likely reflects capture of the ventricular tissue. Although it would be appropriate to strive to achieve selective HBP, which is more physiologic than non-selective HBP, the latter could also be an acceptable endpoint.
His Bundle Pacing Versus Right Ventricular Pacing
There is now ample evidence in the literature that right ventricular apical pacing can be associated with electrical and mechanical ventricular dyssynchrony and adverse remodeling, potentially leading to left ventricular dysfunction, functional mitral regurgitation, left atrial dilatation, atrial and ventricular arrhythmias.15,48,49 There have been suggestions that non-apical site of ventricular pacing might portend better clinical outcomes. Although randomized control trials and meta-analyses of these trials have depicted higher left ventricular ejection fractions in patients with non-apical RV pacing compared with apical RV pacing, findings on survival have been rather limited and inconclusive as most studies were not powered to assess survival.7,8 In a large meta-analysis of 14 randomized controlled trials, Shimony et al observed that non-apical RV pacing compared to apical RV pacing, led to improved LVEF at the end of follow-up with a weighted mean difference of 4.27 % (95 % CI 1.15–7.40 %). This improvement in LVEF was more pronounced in patients with ≥ 12 months of follow-up and those with baseline LVEF of ≤ 40–45 %. However, they found that data regarding exercise capacity, functional class, quality of life, and survival were limited and inconclusive, highlighting the need for additional RCTs examining this issue.8 The advent of HBP has provided an alternative to RV pacing. HBP leads to preservation of narrow QRS in patients with baseline ventricular synchrony, and normalization of baseline bundle branch block in a significant number of patients. Many studies have compared RV pacing with His bundle and found encouraging results in favor of HBP.14,16,19–22 As shown in Table 1, HBP was associated at follow-up with significantly reduced left ventricular end diastolic and left ventricular end systolic dimensions, improved LVEF, reduced left atrial dimensions, reduction in NHYA class, improved quality of life, and reduction in hospitalization for heart failure. There was, however, no significant difference in mortality. It has been depicted that HBP when compared with RV apical pacing, results in reduced left atrial anatomical dimensions and better atrial function as a result of a more physiological left ventricular electromechanical activation and relaxation.20
His Bundle Pacing and QRS Normalization in Prior His Purkinje System Block
In 1919 Kaufman and Rothberger were first to describe the longitudinal fascicular dissociation in the His bundle whereby conduction in the His bundle was through tissue pathways originating from the AV node that were pre-destined to become separate bundle branches. In 1971, James et al. examined the fine structure of the His bundle on light and electron microscopy and described the longitudinal partitioning of strands of Purkinje cells collagen spanning into the bundle branches, and formed the anatomic basis for suspecting longitudinal separation of conduction within the normal His bundle.50 In the late 1960–1970s, studies by Narula, Scherlag, and El-Sherif et al. demonstrated normalization of bundle branch disease with HBP in animal models and humans, supporting the concept of diseased tissue manifesting with longitudinal dissociation within the His bundle.38,39,51 This concept has been confirmed by recent clinical reports of permanent HBP resulting in narrow paced QRS in patients with previous bundle branch block. By pacing and reengaging previously latent fascicular tissue causing narrowing of the QRS complexes in such patients suggests intra-Hisian location of majority of infra nodal heart blocks.24 This sets the stage for an exciting option of restoration of electrical cardiac synchrony utilizing pacing lead in the His bundle instead of implantation of a left ventricular lead via coronary sinus.25–30
His Bundle Pacing and Cardiac Resynchronization Therapy
The overall burden of heart failure in terms of incidence, prevalence, mortality, and management cost is significant, and increases with increasing age. In the USA, economic burden of heart failure is the excess of $30 billion annually, and the prevalence of heart failure now exceeds 5.8 million and each year more than 550,000 new cases are diagnosed.52,53 Despite recent advances in diagnosis and treatment of heart failure, 5- and 10-year survival after diagnosis still remains only approximately 50 % and 10 %, respectively, and following hospitalization with heart failure, 30-day re-admission rates are approximately 25 %. In addition, there is proven association of left ventricular dysfunction with an increased risk of sudden cardiac death.53
CRT with left ventricular lead placement via the coronary sinus in patients with heart failure and evidence of cardiac dyssynchrony is now an established adjunctive therapy in these patients. It is associated with independently significant reduction in mortality and heart failure re-hospitalizations, improvement in quality of life, increase in LVEF, reduction in NYHA class, etc. Unfortunately, approximately 30 % of heart failure patients with cardiac dyssynchrony are non-responders to CRT.54 This can be secondary to patient characteristics, progression of intrinsic disease or inability to implant lead in appropriate location because of technical and anatomical challenges. It is worthwhile noting that non-response is a relative phenomenon, as some response can be lackluster with LVEF improvement, but no clinical improvement. Furthermore, difficulty of coronary sinus cannulation, lack of suitable venous anatomy, and phrenic nerve stimulation are some of the additional obstacles that hamper successful CRT. Although direct LV lead placement via epicardial approach is possible, it is a major undertaking, limited by access to appropriate surgical teams,25 and associated with high risk of lead failure over time.
The observation of HBP leading to restoration of narrow QRS in patients with bundle branch block (electrical resynchronization), which is a manifestation of the phenomenon referred to as longitudinal dissociation, generated interest as to whether this could induce mechanical resynchronization and supplant traditional CRT.26 The feasibility of HBP in CRT-indicated patients was first reported by Lustgarten et al. in 2010, whereby 7 of 10 consecutively studied patients normalized their QRS in response to direct HBP.55 Three years later, Barba-Pichardo et al. conducted a similar study, but with clinical outcomes report in 9 out 16 patients with heart failure, LBBB, NYHA III, indication for CRT and implantable cardioverter defibrillator, who failed LV lead placement via the coronary sinus. After an average follow-up of 31 months, LV end-diastolic and end systolic volumes were significantly reduced and LVEF increased from 29 % at baseline to 36 %.25 As shown in Table 2, other studies have reported similar findings and improvements in NYHA class,26–30 as well as some case reports.56–59 In the most recent study by Sharma et al., successful HBP in 95/106 (90 %) of patients divided into Group I (failed LV lead or CRT non-responders) and Group II (primary strategy in patients with complete heart block, bundle branch block, high-pacing burden, and CRT indication) led to improvement in LVEF from 30 % at baseline to 43 % at follow-up (P<0.001), and reduction in NYHA class from a mean of 2.8 to 1.8 (P<0.001). The authors concluded that HBP may be considered as a rescue strategy for failed biventricular pacing and may be a reasonable primary alternative to biventricular for CRT.30 Unfortunately, there is not sufficient data from these studies to determine whether there is a survival benefit.
Challenges of His Bundle Pacing and the Future Directions
HBP is technically a challenging procedure. However, results from high-volume implanting centers suggest that success rates steadily improve with progressive operator experience. This has been helped by the custom-made HBP leads and delivery sheaths, although the need for even better implanting kits remains. Other challenges at implant include failure to map the His bundle, failure to capture the His bundle, as well as lower R-wave amplitudes and high-pacing thresholds when compared with RV pacing. Low R-wave amplitude might raise safety concerns about ability to sense ventricular arrhythmias, while high-pacing thresholds may adversely affect device longevity.28 Long-term performance of the commercially available specialized HBP lead is unknown. There is also no reported experience with extraction of HBP leads. Extraction of small-caliber, fixed-screw leads can by itself be challenging, but with peculiar location of non-stylet-driven HBP leads, the outcomes of extraction remain unknown for now. However, one will anticipate that with the available contemporary extraction equipment, such a challenge should be feasibly overcome. Publication of any extraction case reports or series in the area of HBP should be encouraged. Although short- to medium-term studies have been re-assuring, concerns remain about proximal conduction disease progressing to infra-Hisian block in His bundle-paced patients. Most studies in the field of HBP have been case series with rare exceptions, even if some of these had comparative groups. Therefore, there is a need for randomized controlled trails in this field before any firm recommendations can be made with a certain degree of comfort in future pacing guidelines. The current HBP Versus Coronary Sinus Pacing for Cardiac Resynchronization Therapy (HIS-SYNC) study, which is comparing the effectiveness of physiologic pacing from a His bundle lead position versus the standard LV pacing with lead in the coronary sinus branches in subjects with heart failure undergoing CRT, will hopefully help to provide more clarity. Recently, recommendations from ‘A Multi-Center HBP Collaborative Working Group For Standardization Of Definitions, Implant Measurements And Follow-Up’ has been published.46 This was a collaboration between several implanters with significant experience in HBP to establish a uniform set of definitions encompassing the different forms of HBP as well as define a standardized approach to gathering data endpoints to ensure consistency in reported outcomes.
Conclusion
HBP is feasible, safe, and efficacious. It offers a superior ventricular synchronous pacing strategy and better outcomes with respect to LV end diastolic and end systolic diameters and systolic function, shorter interventricular electromechanical delay, improvement in quality of life, reduction in NYHA class, and improvement in 6-min walk when compared with RV pacing. HBP can potentially provide more definitive electrical resynchronization and can be considered in heart failure patients with wide QRS who are non-responders to bi-ventricular pacing or those with failed transvenous-coronary sinus LV leads, or may even be employed as a primary CRT pacing strategy pending the results of an ongoing trial. However, implantation can be technically challenging, and this modality has not yet been subjected to the rigor of randomized trials to determine its long-term efficacy and safety compared with traditional pacing strategies.