Atrial fibrillation (AF) is the most common clinically relevant supraventricular arrhythmia. AF is a leading risk factor for stroke and accounts for about one-third of all ischemic cerebrovascular events.1 The last two decades have witnessed a paradigm shift in the management of AF with the development of catheter ablation and improvements in anticoagulant therapies. In this review, we discuss gender-related differences and disparities in the pathophysiology, clinical presentation, and management of AF.
Gender Differences in the Pathophysiology of AF
Men are more susceptible to the development of AF. However, since women live longer than men, the cumulative lifetime risk of AF is similar in men and women, at about 30 %.2 On average, women develop AF 10 years later than men.2 Differences in atrial effective refractory period (ERP) in response to rapid atrial pacing have been reported in men and women. The degree of shortening of atrial ERP was significantly less in premenopausal women compared with postmenopausal women and age-matched men, suggesting the protective role of estrogen.3 In addition, non-pulmonary vein triggers are more frequent in women with AF compared with men.4 More recent evidence points to genetic disparities in ion channel expression between men and women. Ambrosi et al. investigated the mRNA expression of 89 ion channel subunits, calcium handling proteins, and transcription factors important in cardiac conduction and arrhythmogenesis.5 Gender-specific analysis showed lower expression levels in transcripts encoding for Kv4.3, KChIP2, Kv1.5, and Kir3.1 in the failing female left atrium compared with the male left atrium. Gender differences in autonomic control of the cardiovascular system have been described as well. Sympathetic-mediated responses predominate in men, while women have higher degrees of parasympathetic activation, which has been associated with an increased propensity of AF due to extensive vagal innervation of the atrial muscle sleeves extending into the pulmonary veins.6
Morphologically, significant gender-based differences in AF-related atrial remodeling have been observed. Fibrotic remodeling of the left atrium leads to electrical dissociation of atrial cells that contributes to higher incidence and recurrence rates of AF. Li et al. analyzed tissue samples from men and women with long-standing, persistent AF and showed that women have a significantly higher degree of fibrotic remodeling compared with men.7 This morphological difference was driven by differential expression of fibrosis-related genes and proteins, such as transforming growth factor-beta, which were upregulated in women with persistent AF. Cochet et al. reported that female gender was independently associated with delayed gadolinium enhancement in patients with AF, as well as in patients with no AF or structural heart disease.8 In a subanalysis of the AF Follow-up Investigation of Rhythm Management (AFFIRM) trial, female gender was significantly associated with higher rates of left atrial remodeling and adverse cardiovascular endpoints.9
Gender modulates how various risk factors contribute to AF.10 Obesity appears to impart a higher risk of AF in men compared with women (hazard ratio [HR] per standard deviation increase 1.18; 95 % CI [1.12–1.23] in women versus 1.31; 95 % CI [1.25–1.38] in men; Pinteraction<0.001). Women with AF have a lower prevalence of coronary disease and sleep apnea compared with men. However, hypertension and heart failure with preserved ejection fraction are more prevalent in women with AF, likely reflecting the later age of onset.
Gender Differences in the Clinical Presentation of AF
Substantial differences in clinical symptomatology of AF exist between men and women. In the Outcomes Registry for Better Informed Treatment of AF (ORBIT-AF) registry, women with AF experienced more symptoms and worse quality of life in comparison with men.10 Similarly, in the Euro Observational Research Program on AF (EORP-AF) pilot survey, women experienced a significantly higher rate of palpitations and fear and anxiety compared with men.11 A similar pattern of AF-related symptoms was also reported in the Prevention of Thromboembolic Events European Registry in AF (PREFER in AF) registry. In this analysis of 7,243 patients, 95 % of women with AF were symptomatic compared with 90 % of men.12
In addition to variations in symptomatology, there are important prognostic differences between women and men with AF. A meta-analysis of 30 studies from 1996 to 2015, including >4 million participants, indicated that female gender is an independent risk factor for all-cause and cardiovascular mortality, incident heart failure, and stroke in patients with AF.13 There are significant disparities in AF-related stroke risk, with women experiencing more strokes as well as more disabling strokes compared with men, as discussed below.
Gender Differences in AF Management
Rate Control
Gender bias is apparent in the choice of medications for rate control of AF. In the ORBIT-AF registry, women were less likely to receive beta-blocker therapy (62.0 % versus 65.5 %) and were more likely to receive digoxin (24.6 % versus 22.6 %).10 In the EORP-AF registry, use of digoxin as a rate-control agent was significantly more common in women (25 % versus 19.8 %), while there was no difference in prescription rates of beta-blockers.11 In the Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in AF (ROCKET-AF) trial, digoxin was used in 42 % of female participants compared with 38 % of males, and digoxin use was associated with increased all-cause mortality, vascular death and sudden death.14 Other studies have also revealed an association between digoxin and higher rates of cardiac death.15 In this context, the consistently higher rates of use of digoxin as a rate-control agent is women is concerning. Whether this reflects poor tolerability to commonly used rate-control medications is unknown.
Gender-specific differences also appear to exist in the use of non-pharmacological rate-control measures. In the ORBIT-AF registry, women had significantly higher rates of atrioventricular nodal ablation and pacemaker implantation (adjusted HR 1.97; 95 % CI [1.30–2.97]) compared with men over a median follow-up of 2.3 years.10
Rhythm Control
Gender differences in rates of prescription of anti-arrhythmic medications for AF have been debated. In the EORP-AF survey, rhythm control was less commonly utilized in women despite a higher rate of symptomatic AF and lower quality of life. Rates of electrical cardioversion were 18.9 % in women compared to 25.5 % in men.11 In the PREFER in AF observational cohort, women were more likely to receive pharmacological cardioversion while men had higher rates of electrical cardioversion.12 However, in the ORBIT-AF registry, there was no difference in rates of anti-arrhythmic medication use in women compared with men.10 In a nationwide analysis of all in-patient cardioversions in the US, we have previously reported that in-hospital rates of electrical cardioversion were significantly higher in men compared with women (58.4 % versus 48.6 %).16 Rates of AF recurrence following cardioversion have also been reported to be higher in women.17
In this context, it is important to understand that women appear to have a higher risk of side-effects with rhythm-control strategies. Women with AF on Class IA and Class III anti-arrhythmic medications have higher rates of torsades des pointes and bradyarrhythmias requiring a pacemaker. In the Fibrillation Registry Assessing Costs, Therapies, Adverse Events, and Lifestyle (FRACTAL) registry, Essebag et al. have reported that female gender was an independent risk factor that determines the need for a pacemaker in patients taking amiodarone for AF.18
Catheter Ablation
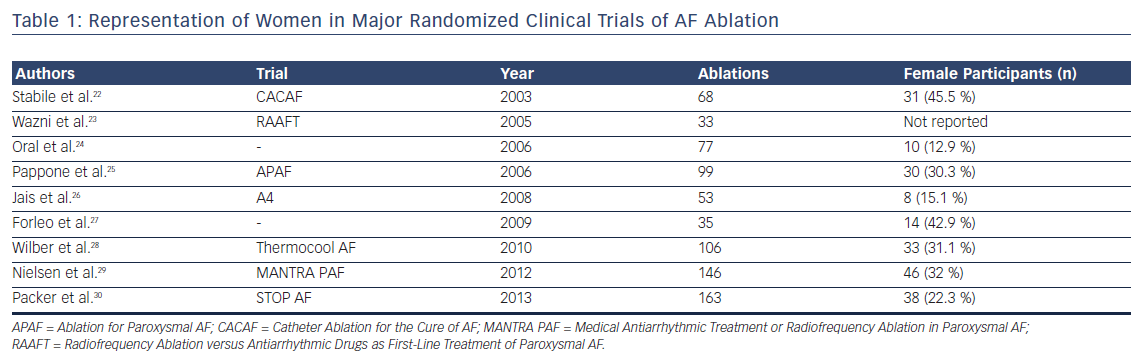
Catheter ablation has emerged as an important therapeutic strategy in the management of AF. Significant gender disparities in utilization of catheter ablation, in which women are referred late and less frequently for catheter ablation of AF compared with men, have been identified. Women referred for ablation are older and have larger indexed left atrial dimensions.19 In a nationwide analysis of AF ablation procedures, women were 17 % less likely to undergo catheter ablation compared with men.20 Another analysis of AF care strategies in Medicare beneficiaries demonstrated significantly lower referral rates for catheter ablation in women (HR 0.65; 95 % CI [0.63–0.68]), even after adjusting for multiple confounding variables.21 There is similar under-representation of women in randomized trial data of AF ablation (Table 1). A meta-analysis of all AF ablation clinical trials reported that women constitute only one-fifth of the study population.31
In addition to disparities in utilization, gender-based differences in efficacy and safety of AF ablation also exist. Women suffered from a higher risk of complications after AF ablation in multiple studies. Patel et al. reported that women undergoing catheter ablation more often had persistent AF, a higher proportion of non-pulmonary vein triggers, lower ablation success rates, and significantly higher complication rates, the latter driven primarily by vascular complications.32 In a nationwide analysis of AF ablation complications, overall, women had higher in-hospital complication rates than men (7.51 % versus 5.49 %; p<0.001).33 A more recent study reported that women undergoing AF ablation had a higher risk of vascular-related complications, hemorrhage, and perforation or tamponade, and that overall, women had an increased risk of all-cause hospitalization compared with men (9.4 % versus 8.6 %; p=0.07).34
Various patient-related factors could explain the higher rates of vascular and hemorrhagic complications in women. Female patients tend to have smaller vessel calibers compared with men, which may increase the risk of vascular injury. In AF ablation studies, women have been shown to have higher activated partial thromboplastin times compared with men, even when lower doses of heparin are administered.35 However, although these are interesting hypotheses, the underlying mechanism leading to higher complication rates in women is yet to be deciphered.
Gender Differences in Stroke Risk
AF is a well-recognized risk factor for stroke. In a retrospective Swedish AF cohort study of 100,802 patients with AF, female gender was an independent risk factor for stroke (HR 1.18; 95 % CI [1.12–1.24]) even after adjusting for multiple confounding variables.36 As described earlier, women with AF tend to have larger left atrial volumes and reduced atrial contractility compared with men, which can increase the risk of atrial thrombi. Elderly postmenopausal women also have higher rates of diastolic dysfunction and elevated systolic blood pressure compared with men, which can lead to accelerated cardiovascular remodeling and endothelial dysfunction that translates into a higher risk of stroke.37
Efficacy of Anticoagulants
For many decades, warfarin was the choice of anticoagulant for stroke prophylaxis in AF. Direct-acting oral anticoagulants (DOACs) are now available and have been shown to be non-inferior in stroke prevention. Analysis of trial data demonstrates some gender-specific differences in efficacy and risk of bleeding with the use of these medications.
Warfarin
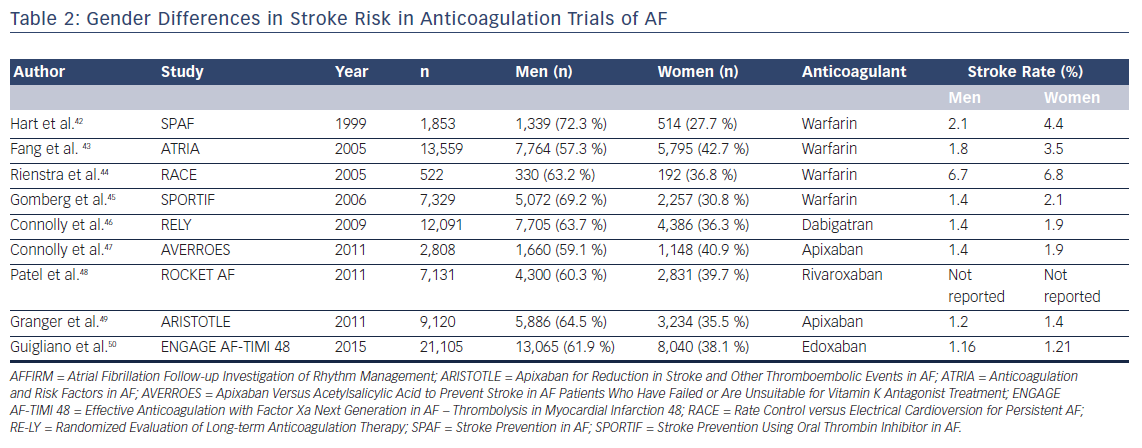
A meta-analysis of five randomized controlled trials of patients with AF reported that warfarin reduces the risk of ischemic stroke by 84 % (95 % CI [55–95 %]) in women compared with 60 % (95 % CI [35–76 %]) in men.38 However, more recent evidence indicates that women have a higher residual stroke risk compared with men receiving oral anticoagulation. In a post hoc analysis of the AFFIRM trial, Sullivan et al. reported that women were at greater risk of ischemic stroke than men despite similar anticoagulation patterns.39 The difference in ischemic stroke risk was primarily related to a higher proportion of women being outside the therapeutic range for warfarin. Time in therapeutic range is recognized as a major factor determining stroke risk in AF patients on warfarin. A recent meta-analysis also reported similar findings, showing that female patients with AF on warfarin had a significantly higher residual risk of stroke and systemic thromboembolism than men (OR 1.28; 95 % CI [1.11–1.47]).40
DOACs
Gender-based differences in stroke risk are less obvious in trials of DOACs. A meta-analysis of 71,683 participants included in the ROCKET-AF, Randomized Evaluation of Long-term Anticoagulant Therapy (RE-LY), Apixaban for Reduction in Stroke and Other Thromboembolic Events in AF(ARISTOTLE), and Effective Anticoagulation with Factor Xa Next Generation in AF – Thrombolysis in MI 48 (ENGAGE AF-TIMI 48) trials showed no gender-based differences in stroke or bleeding risk among patients assigned to DOACs.41 In contrast to patients assigned to warfarin, the risk of residual ischemic stroke among those assigned to DOACs did not reveal any gender bias.
In summary, although prior trials of stroke prevention in AF revealed a higher rate of ischemic stroke in women with AF on warfarin, DOACs appear to not suffer from this gender-based difference in efficacy (Table 2).
Conclusion
Multiple studies have shown major gender-based differences in the clinical profile and management of AF. Whether these are related to differences in biology or represent treatment disparities is unknown. This area of cardiac electrophysiology deserves further study.