Although less common than other forms of cardiomyopathy, cardiac sarcoidosis (CS), both as part of a systemic process and in its isolated form, is an important, and increasingly recognized disorder. This is felt to be due, in part, to advances in cardiac imaging and heightened awareness of the disorder.1,2 CS is associated with high rates of morbidity and mortality, including sudden cardiac death. The early recognition of CS and prompt initiation of treatment is of the utmost importance. Generally a systemic process, sarcoidosis is an idiopathic, inflammatory, granulomatous disorder generally involving the lung and lymph nodes, but it can also occur in ocular, cutaneous, neurological, musculoskeletal, and cardiac forms.
The incidence of systemic sarcoidosis varies among populations, with rates ranging from 10 per 100,000 in a primarily white group in Minnesota, to 30 and 39 per 100,000 in African–American men and women, respectively, in a more racially mixed population.3,4 Overt cardiac disease occurs in 2.3–7% of patients with systemic disease, however, autopsy studies and studies in patients undergoing cardiac MRI show evidence of CS in about 25% of patients with systemic sarcoidosis.5–9 Isolated CS, with disease determined to be localized to the heart based on clinical and imaging characteristics, may have a more malignant course.2 A rare subgroup of patients with CS has a rapidly progressive, fulminant course characterized by intractable congestive heart failure (CHF) with limited response to therapy.10 These patients may require cardiac transplantation.
Patients with CS may present with classic symptoms of CHF, such as dyspnea, orthopnea, and fatigue, along with signs, such as peripheral edema and rales. They can also have arrhythmic symptoms, such as palpitations, light-headedness, or syncope, due to involvement of the cardiac conduction system. A high index of suspicion for CS by health care providers is important as treatment may need to be started swiftly.
Diagnosis
Several guidelines for the diagnosis of CS have been published.11-13 The first step in the diagnosis of CS is a thorough history and physical examination. Symptoms of mild light-headedness can be of critical importance for the identification possible arrhythmias. ECG is key for detection of conduction disturbances such at right bundle, bifasicular, or varying degrees of atrioventicular (AV) block. In addition, frequent premature ventricular contractions (PVCs) may be seen. Unexplained advanced AV block or ventricular tachycardia (VT), particularly in younger patients, may be important indicators of CS.14,15 Chest X-ray or computerized tomograhy (CT) of the chest are used for the detection of hilar or mediastinal lymphadenopathy. Laboratory tests may demonstrate elevation of angiotensin-converting enzyme levels, as well as hypercalemia or hypercalciuria, particularly in patients with systemic disease. However, these test are somewhat non-specific, and cannot be relied on for the diagnosis or exclusion of CS.
Cardiac echocardiography is another important element in the evaluation of the patient with suspected CS. Characteristic findings include reduced left or right ventricular function, wall motion abnormalities not in the normal distribution of the coronary arteries, aneurysmal segments, or thinning of the basal portions of the septum. Mid and basal septal hypertrophy can be seen, and may mimic hypertrophic cardiomyopathy. Valvular abnormalities, particularly mitral regurgitation, may be present if the infiltrative process affects the papillary muscles.
Occasionally, single photon emission computed tomograhy (SPECT) myocardial perfusion imaging may be useful indentifying patients with CS. Typical finding are fixed defects suggesting scarring, particulary when localized to the base of the septum or lateral wall.
Ambulatory event recording or Holter monitoring are important in screening for arrhythmias, particularly in patients with symptoms of light-headedness or near syncope.
Although obtaining cardiac tissue for analysis is considered the gold standard for the diagnosis of CS, the yield of endomyocardial biopsy is low, 19.2% in a study of 26 patients by Uemura et al.,16 but may be improved when biopsy is coupled with electroanatomic maping.17
Biopsy of extracardiac sites where sarcoidosisis suspected remains the primary method of diangnosis for most patients. This may be accomplished by mediastinoscopy with lymph node biopsy or endobronchial ultrasound-guided needle biopsy.
Coronary angiography may be important to rule out an atypical presentation of coronary heart disease.
Advanced Cardiac Imaging
Multimodality cardiac imaging using a team approach involving cardiac imagers and radiologists is the key to best diagnosis and management of CS, and is an example of how cardiac MRI and PET may be complementary.
Advanced cardiac imaging is suggested for patients with extracardiac sarcoidosis with symptoms of CHF, possible arrhythmia, along with an abnormal ECG and/echo.18
Patients without known extracardiac sarcoidosis but with new unexplained CHF associated with conduction disturbances such as AV block, or ventricular arrhythmias, such as ventricular tachycardia, may also be candidates for advanced imaging.
Cardiac MRI
For most patients, cardiac MRI with the use of late gadolinium enhancement (LGE), is the favored method of initial diagnosis of CS.19 This is primarily due to its superior spatial resolution, which allows improved sensitivity to detect and localize even small areas of fibrosis characteristic of CS. Cardiac MRI findings include focal, bright, patchy areas of LGE, particularly at the base of the anterior septal, inferior or lateral walls, in a non-coronary distribution. Other findings are papillary muscle or right ventricular LGE, areas of wall thinning, left ventricular aneurysms, or septal hypertrophy.
In addition to its diagnostic use, cardiac MRI may yield important prognostic information. A prospective, observational study by Greulilch et al. followed 155 patients with systemic sarcoidosis who underwent cardiac MRI, of which 153 were available for follow-up (median follow-up time 2.6 years).9 LGE was present in 25% (n=39) of patients and 28% (n=11) of this group experienced one or more of the hard endpoints of death, aborted sudden cardiac death, or appropriate ICD discharge as opposed to only one patient who died among the 114 patients without LGE.
Murtagh et al. followed 205 patients with extracardiac sarcoidosis who underwent cardiac MRI as part of their evlaution and who had an EF>50%.20 20% of patients (n=41) had evidence of LGE. Over a mean follow-up period of 36±18 months, 24% (10/41) experienced death/sustained VT. Patients with LGE had a 20-fold higher risk of adverse events compared to those without LGE. Scar burden was the most important predictor of events. Ekstrom followed 59 patients with CS as defined by Heart Rhythm Society crieteria who underwent cardiac MRI. 39% (n=23) reached the primary endoints, which was either death, transplant, or life threatening arrhythmias. The median time of follow in this subgroup was 15 months. They found that the extent of LGE as a percentage of left ventricular mass was the most important predictor of events, with LGE >22% having best predictive value.21
Quantitative assessment of myocardial tissue characteristics using T1 and T2 mapping techniques can be a useful adjunct to observations of LGE alone when diagnosing CS. In a study by Greulich et al., 61 patients with systemic CS had higher median elevations of native T1 and T2, higher extracellular volume, and lower post-contrast T1 compared with 26 healthy controls.22 These findings were present even in patients without LGE, suggesting it could be used to detect early cardiac disease. A study by Puntman et al. of 53 patients with systemic sarcoidosis, yielded similar findings of higher native T1 and T2 compared with controls.23 Follow-up cardiac MRI performed on 40 patients demonstrated a significant reduction in native T1 and T2 levels in patients receiving anti-inflammatory treatment compared with those who did not.
PET
Improvements in imaging and patient preparation techniques, as described in recent consensus statements, along with an evolving body of data, have brought PET imaging to the forefront for both initial diagnosis and management of CS.18,24 Pooled estimates of accuracy of PET for the diagnosis of CS from a meta-analysis of seven studies has demonstrated sensitivities of 89% and specificities of 78%.25
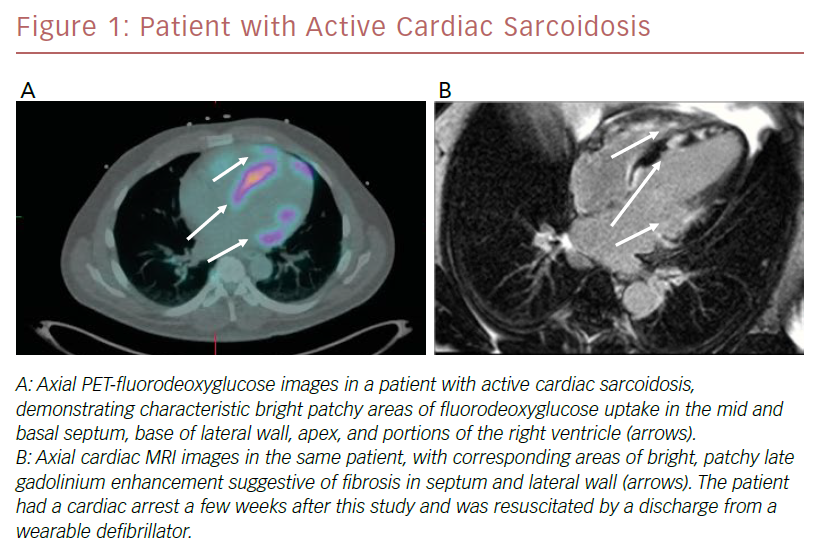
PET imaging is based on the principle of uptake of the glucose analog FDG in areas of intense inflammation, characteristic of active sarcoidosis. Lack of uptake of radioisotope suggests extensive fibrosis, inactive disease, or successfully treated disease. PET images are acquired and registered to CT images for co-localization. Rest myocardial perfusion imaging, generally performed with RB-82 or 13N-ammonia, is also performed to detect areas of decreased activity suggestive of acute inflammation or scarring. A key component to optimal imaging is meticulous adherence to a pre-procedural diet that is high in protein and fat and very low in carbohydrates, followed by at least a 4–12-hour fast.26 The resultant low glucose, low insulin, high fatty acid milieu suppresses uptake of glucose in area of normal myocardium and enhances imaging of areas of inflammation. PET imaging is preferred for patients with relative contraindications to MRI, including those with older cardiac implantable electronic devices, renal insufficiency, obesity, or claustrophobia. An example of a patient who underwent both PET/FDG and cardiac MRI as part of an evaluation for CS is shown in Figure 1.
It should be noted that the types of abnormalities appearing on either cardiac MRI and/or PET are not completely specific to CS; the differential diagnosis includes myocarditis in its various forms, including giant cell, lymphocytic, and eosinophilic,27 as well as arrhythmogenic right ventricular cardiomyopathy.28 Further, physiologic uptake of FDG, particularly in the lateral wall of the left ventricle, may occur in normal and abnormal patients, despite best efforts to suppress with pre-procedural diet protocols.
Specific findings on PET imaging can provide important prognostic information. In a study of 118 patients undergoing PET for evaluation of CS, Blankstein et al. identified specific PET imaging patterns associated with death or sustained VT.29 They found annualized event rates of 7.3% in patients with normal perfusion and metabolism, 18.4% in patients with either perfusion or metabolic abnormalities, and 31.9% in patients with perfusion and metabolic abnormalities (median follow-up 1.5 years).29 Patients demonstrating right ventricular uptake of FDG had an annualized event rate of 55.2%.
Combination of Cardiac MRI and PET
A good approach to the management of CS may use both techniques: cardiac MRI to make the initial diagnosis and assess prognosis and PET to document an active inflammatory process as well as to further assess prognosis and monitor response to therapy. Further, PET may be useful in cases of an indeterminate cardiac MRI and vice versa.
Vita et al. used an imaging rubric combining cardiac PET and MRI findings based on specific imaging characteristics to develop the likelihood criteria for the diagnosis of CS.27 They then compared these findings to a pre-specified reference criteria based on clinical data and consensus statement recommendations. Combination imaging helped reclassify 48 (45%) of the patients into higher or lower probabibility categories, most of them (80%) being correctly re-classified when compared with final diagnosis. This reclassification had important clinical implications, with initiation or modification of immunosuppressive therapies being more likely among patients in the higher probability groups.30 Wicks et al., following 51 consecutive patients with suspected CS as defined by Japanese Ministry of Health and Welfare criteria (JMHW), found superior sensitivity for the diagnosis of CS using simultaneous imaging with a hybrid PET/MRI scanner compared with stand-alone PET or MRI.31 This study also provided important prognostic information, demonstrating 18 (35%) adverse events over the median follow-up of 2.2 years. Of the 17 patients with abnormalities on both PET and MRI, 71% (n=12) had adverse events.31
Medical Therapy
Immunosuppressant therapy remains the mainstay of treatment for CS and is longstanding practice despite incomplete validation and lack of randomized clinical trials.32 Although there are no formal guidelines for medical treatment of CS, an expert consensus statement11 and treatment protocols from major sarcoidosis centers have been published.1,33–35 Survival or improvement in LVEF with corticosteroid therapy has been demonstrated in some studies, but not others.33,36,37 Treatment protocols vary between centers, but generally prednisone, initially at higher doses (40 mg/day) is initiated, then gradually tapered over 6–12 months, depending on clinical response. Methotrexate, along with folic acid, may be added if clinical response inadequate, or if evidence of persistent inflammation on follow-up PET. Methotexate may also permit down titration of prednisone dose. Azathioprine, mycophenolate, or hydroxychloroquine may also be added as adjuncts or used as alternatives, to either allow reduction in dose of prednisone, or when clinical improvement is not seen.1,34 It is strongly recommended that treatment be managed by healthcare providers and pharmacologists familiar with these medications in dedicated clinics, to minimize the high potential for complications. Patients with CHF should be treated with standard guideline-directed medical therapy including beta blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and aldosterone blockers. Antiarrhythmic agents such as amiodarone are used to maintain normal sinus rhythm in patients with atrial arrhythmias or symptomatic VT. Anticoagulation should be strongly considered in patients with risk factors for stroke.
Although there are no standardized protocols for follow up, cardiac PET may be performed 3-6 months after initiation of immunosuppressant therapy to assess response.18,24,35 The degree of cardiac inflammation can be quantified by measurement of the specific uptake value of FDG in areas of myocardial uptake.24 Evidence of reductions in inflammation using these measurements have been shown to correlate with improvements in ejection fraction.38
If evidence of decreased inflammation is observed, immunosuppression may be slowly tapered off over a period of months. Once this is accomplished, a chronic suppressive dose of 5–10 mg prednisone daily can be prescribed. If inflammation persists, the addition of other immunosuppressant agents can be considered. PET imaging may again be performed 3–6 months later to reassess response. Once clinical stability is achieved, a reasonable approach is to follow patients using echo to monitor for relapse. This may be done every 6–12 months. It is important that patients receive careful follow-up by a team that is familiar with managing this disorder, in a dedicated clinic if available.
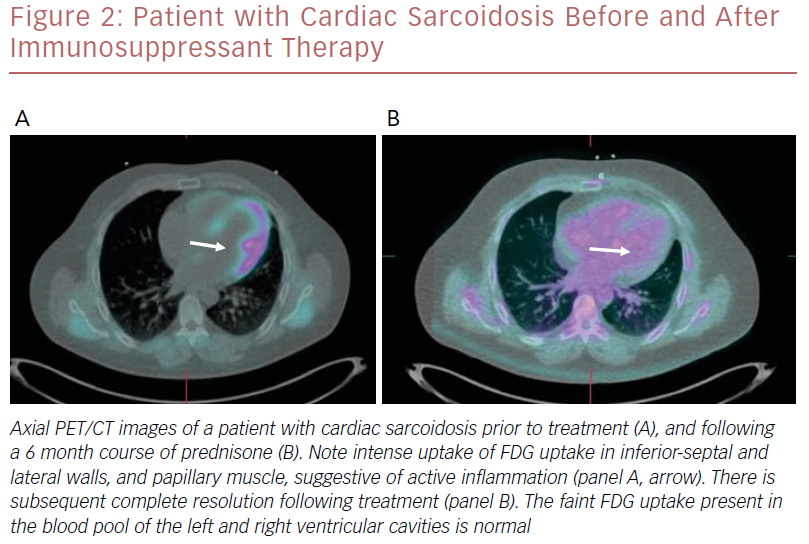
An example of a patient who had a good response to immunosuppressant therapy is shown in Figure 2.
Interventional Therapy
Patients with CS are at high risk for sudden cardiac death (SCD). Episodes of non-sustained and sustained VT with syncope are common. In a study of 45 patients with CS and an ICD by Betensky et al., 37.8% (n=17) had a least one important arrhythmic event requiring therapy during a mean follow-up time of 2.6±2.7 years after device implantation. Notable in this study was the finding that a significant number of serious events occurred in patients with a LVEF >35%, higher than that generally recommended for placement of an ICD for primary prevention of sudden cardiac death in patients with other types of cardiomyopathy.39 Patients with isolated CS are at particularly risk for arrhythmias. Kron et al. reported on a retrospective multicenter review of 235 patients with CS and ICD in place. Of this group, 5.5% of patients (n=13) were felt to have definite or suspected isolated CS. Over a mean follow-up of 4.2 years, 69% (n=9) of these patients had appropriate ICD therapy, a much higher percentage than patients with CS as part of a systemic process, 33.8% (n=222).40 In 2014, the Heart Rhythm Society, in conjunction with six major cardiology organizations, published an expert consensus statement regarding diagnosis and management of patients with arrhythmias associated with CS. Included are recommendations for diagnosis, screening, management of conduction abnormalities, management of atrial and ventricular arrhythmias, and recommendations for ICD implantation.11
Prognosis
Prognostic information regarding CS differs due variations in the evidence base regarding definitions, diagnosis, degree of cardiac involvement, treatment protocol used, and use of current guideline-directed electophysiologic and medical therapy for CHF. Earlier studies suggested a poor outcome, particularly in patients with reduced LVEF or patients who did not receive immunosuppressant therapy.36,37 More recent studies, using aggressive treatment protocols and advanced cardiac imaging, suggest a much better prognosis. Kandolin’s important study of 110 Finnish patients with CS followed over a period of 25 years, reported transplant-free survivals of 97% at 1 year, 90% at 5, and 83% at 10 years.1 The subgroup of patients who presented with CHF had somewhat worse outcomes, with transplant-free survivals of 90%, 75%, and 53% at 1, 5, and 10 years, respectively. This series was also notable for aggressive treatment with immunosuppressant and judicious use of ICDs among patients, along with advanced cardiac imaging to monitor the condition.
A retrospective study by Zhou et al. of 73 patients with CS, also receiving state of the art care, demonstrated survivals of 95% at 5 years, and 93.4% at 10 years.41
Conclusion
CS is an important and increasingly recognized cause of cardiomyopathy, both as part of a systemic process or isolated to the heart. It is characterized by elements of CHF, cardiac conduction disturbances and potentially fatal arrhythmias. Optimal diagnosis and treatment rely strongly on a high clinical index of suspicion. Cardiac imaging with cardiac MRI and PET is key for best diagnosis and monitoring of therapy. Although data are inconclusive, immunosuppression may be useful in improving symptoms, prognosis and LVEF. Judicious use of ICDs, even in patients with LVEF >35%, can be lifesaving. Finally, it should be emphasized that strong collaboration among cardiologists, cardiac electrophysiologists, cardiac imagers, radiologists and pulmonologists is of the utmost importance for the optimal care of patients with this complex condition.