Advanced heart failure (AHF) affects approximately 5–10 % of the current 6.6 million people living with heart failure, having a higher incidence in those above 65 years of age.1 The presence of the disease is associated with 50–80 % mortality at 1 year, indicating the need for innovative therapies to manage the burden of this disease. Heart transplantation is the gold standard for AHF, although limitations exist due to the availability of donor hearts; only ~4,500 orthotopic heart transplants are performed worldwide each year.2 Mechanical circulatory support offers the potential to restitute ventricular function, improve overall functional capacity and quality of life. This strategy, however, is not free from complications and device-related issues make this therapy a challenge to apply to a broader population.
The era of mechanical circulatory support began in the 1950s with the successful management of post-cardiotomy syndrome. The favorable outcomes led to new applications including their use as a bridge to heart transplantation (BTT) and, by 1994, the US Food and Drug Administration (FDA) gave approval for pneumatically-driven left ventricular assist devices (LVADs) as BTT.3 Soon after, electrically-driven LVADs where developed, and by 1999 Columbia University had reported its experience of 95 patients supported by HeartMate XVE for 108 days and eventually transplanted.4
The increasing prevalence of heart failure and the limitations of organ donor availability for heart transplantation led to LVADs being used as a permanent therapy in AHF patients who were not candidates for transplant. The Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial investigated LVADs as long-term myocardial replacement therapy or destination therapy (DT).5 This trial randomized New York Heart Association class IV patients with left ventricular ejection fraction ≤25 % to LVAD versus optimal medical therapy. Greater survival was shown in those receiving LVAD therapy at 1 year (52 % versus 25 %), allowing the FDA to approve LVADs as a DT. Moreover, improvements in quality of life, depression and functional status were significant in the LVAD cohort.5 The first generation of LVADs featured pulsatile flow (PF), simulating the native pulsatile function of the heart, but the size of the device requiring implantation in the abdominal cavity limited its application to male patients in most cases. Furthermore, its multiple components tended to fail, it required replacement after 18 months, and frequent adverse events were noted including bleeding, stroke and device infection. Innovations in pump design with a smaller, single high-speed rotary impeller, providing continuous flow (CF) offered long-term durability. A randomized controlled trial in DT patients compared the outcomes of both flow profiles (PF versus CF).6 The CF group showed greater survival (58 % versus 24 %) at 2-year follow up. Furthermore, freedom from disabling stroke and reoperation for device malfunction was higher in the CF-LVAD compared to the PF device (11 % versus 46 %).6
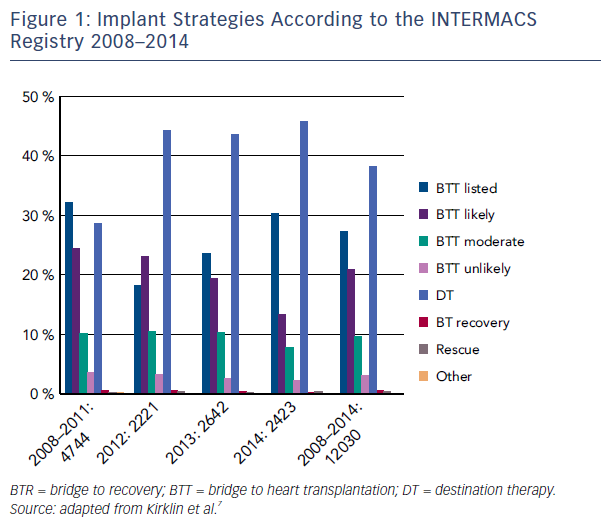
The favorable outcomes seen in these clinical trials of CF-LVAD made the therapy a feasible option for many AHF patients. The most recent Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) report shows that survival continues to improve, now being 80 % at 1 year and 70 % at 2 years.7 These results are consistent with post market approval data showing survival of 83 % and 75 % at 1 and 3 years, respectively.8 Not surprisingly, contemporary registry data report that CF pumps account for all DT implants since 2010 (see Figure 1).7
Matching Patients to Devices
There are two current FDA-approved LVAD therapies: HeartMate II, which uses axial flow, and HeartWare, which uses centrifugal flow. The former has been approved as DT and BTT, while the latter is approved for BTT only. Selecting appropriate LVAD therapy for each patient is difficult, as the decision has to be considered against the complications and their impact on outcomes. The INTERMACS profile provides a guideline for risk-stratifying potential recipients of mechanical circulatory support.9 In a study by Boyle et al. using INTERMACS data, 101 patients on CF pumps were divided into three categories: cardiogenic shock (group 1), inotrope dependent (group 2) and ambulatory AHF (group 3).10 Survival at 36 months for group 1 versus 3 was lower (51.1 % versus 95.8 %, P=0.011). it was found that patients with INTERMACS profiles 1–3 were associated with longer hospital stays compared to INERMACS profiles 4–7.10 Although recent registry data show that patients with profiles 1 and 2 remain the predominant population being implanted, there has been a trend since 2012 for more patients with INTERMACS profiles 3 and 4 to receive implants.7 This change is likely due to post FDA approval studies in DT patients demonstrating superior 2-year survival in those with profiles 4–7 (who are not inotrope-dependent) compared to profiles 1–3 (67±6 % versus 59±4 %).11 Thus, the timing of implantation before further disease progression is important. Many AHF patients have clinical signs and symptoms that portend a further decline in health status, including medication intolerance, frequent heart failure-related hospitalizations and end-organ dysfunction (i.e. hepatic and renal). When these ominous signs develop, prompt referral to an AHF specialist should be considered, as patients with a higher risk of mortality may benefit from mechanical circulatory support therapy.
Predicting Risk
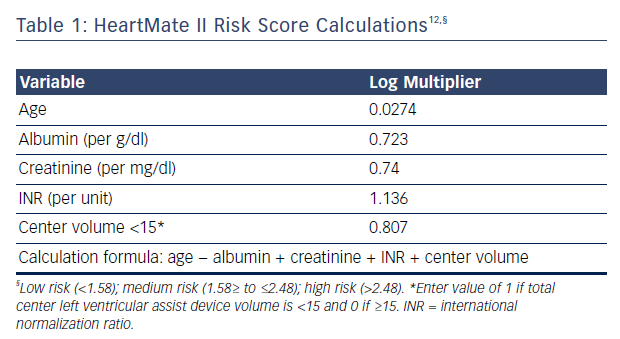
Applying long-term device therapy to AHF patients can be associated with an increased risk of perioperative mortality and poor outcomes. Recent changes in implantation strategy reflect the increased understanding of patient selection, perioperative management and long-term care. Risk stratification prior to implantation is thus critical for good outcomes. The HeartMate II risk score has been developed using large multicenter clinical trial data assessing 90-day mortality, see Table 1.12 The study included >50 % of patients receiving devices as DT in both derivation and validation groups. The HeartMate II risk score cutoffs were 1.58 for low risk, 1.58 to ≤2.48 for medium risk and ≥2.48 for high risk. The receiver operating characteristic curve in the total sample (derivation plus validation) was 0.71 (95 % CI [0.66–0.75]) and mortality in the validated cohort was 8 %, 11 %, and 25 %, for the low, medium and high-risk groups, respectively.12 Of the risk factors identified, age and center experience were determinants of long-term survival for patients implanted as DT (>12 months post implant).12 The model has limitations, however, as its has been shown to poorly discriminate 90-day mortality after LVAD implant when applied to other AHF cohorts and fails to discriminate between low-risk patients who can benefit from LVAD and patients with too high a risk, where LVAD therapy may be contraindicated or futile. Deciding which low-risk patient (ambulatory AHF that is not inotrope dependent) may benefit from this therapy can be a challenge. The Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management (ROADMAP) trial has shown that outcomes in those considered to have lower risk (INTERMACS profiles 4–7) are as favorable as earlier trials, with a survival at 1 year of 80 % versus 64 % in those on optimal medical management.13 Furthermore, improvements in New York Heart Association class 6-minute walk distance, healthrelated quality of life, and depression were more significant in LVAD patients despite having increased adverse events (1.89 events/year) and frequent hospitalizations.13 The study was limited, however, by being a non-randomized observational study and by physicians allocating patients to LVAD therapy at their discretion, inherently introducing bias. Nonetheless, risk prediction can help clinicians start a conversation with patients and caregivers to educate them about the risks and benefits of the therapy. Estimating mortality risk for continuing medical therapy versus LVAD therapy can also help patients understand whether the implantation of a device is in their best interest.
Adverse Events
Despite the meaningful advances in device technology, LVAD continues to be associated with a high rate of adverse events. In the current era of CF-LVAD, only 30 % of patients are free from any major adverse event at 1 year, though this has improved from earlier DT trials.11,14 Device-related complications include stroke, infection, bleeding, pump thrombosis, and ventricular arrhythmias. Non device-related complications include right heart failure. The impact of these complications on mortality is time dependent. In the early phase (<3 months) multiple organ failure is the major cause of death; after 3 months, neurological causes predominate.7 A description of the causes, mechanisms and treatment of each complication goes beyond the scope of this review and has been given by prior authors.15
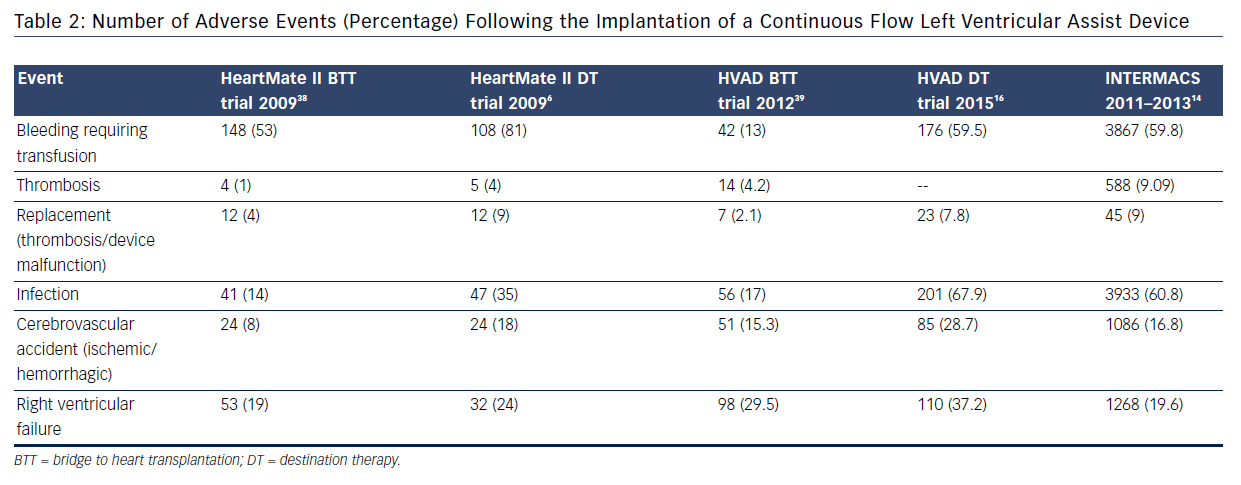
Two recent clinical trials assessing the use of CF technology in DT patients have provided further insight into the burden of complications: ROADMAP13 and ENDURANCE™ (A Clinical Trial to Evaluate the HeartWare Ventricular Assist System).16 The ROADMAP trial was a prospective multicenter non-randomized observational study evaluating the outcomes of initial treatment with LVAD (n=94) versus optimal medical management (OMM, n=103) in ambulatory New York Heart Association IV patients not on inotrope. Individuals in the OMM group were able to transition to LVAD therapy if necessary.13 Within 1 year, adverse events were more frequent in the LVAD group: 47 % due to bleeding (31 % due to gastrointestinal causes), 6.4 % due to pump thrombosis, 8.5 % due to stroke (5.3 % ischemic and 4.3 % hemorrhagic), and 18.1 % due to ventricular arrhythmias; however, worsening heart failure symptoms occurred more often in the OMM group (35 % versus 10.6 %). The composite event rate per patient year for the LVAD group was 1.89 versus 0.83 for the OMM group. Bleeding was the leading cause for rehospitalizations post implant in LVAD; worsening heart failure caused the majority of rehospitalizations in OMM patients. Notably, major causes of death in the LVAD group were sepsis, multiple organ failure, right heart failure, ventricular tachycardia, thrombus and stroke. Despite this, quality of life was better for LVAD patients (55 % versus 23 %, P<0.001). The ENDURANCE trial evaluated 2-year survival and freedom from disabling stroke in patients with HeartWare HVAD versus HeartMate II devices.16 The study demonstrated non-inferiority of the CF centrifugal design over the axial flow device. A higher rate of pump exchange occurred with the axial flow design (16.2 % versus 8.8 %), while a higher stroke rate occurred with the centrifugal flow design (28.7 % versus 12.1 %).16 Although, post approval LVAD DT studies have shown a favorable trend towards a reduction in adverse events and recent registry data support this, the issue of readmissions is a limiting step in the expansion of this technology to a broader heart failure population (see Table 2).
Readmissions
In general, the annual rate of recurrent hospitalizations due to adverse events in CF-LVAD patients is 65 %. The majority of adverse events occur in the first 6 months post implant, with the most common being bleeding (gastrointestinal), cardiac causes (heart failure and arrhythmias), infections, and thrombosis (stroke or pump thrombosis).7,17 The Mayo Clinic findings from 224 admissions in 115 patients followed for 2.3 years supported these findings, with the major causes of hospitalization in the first 6 months being bleeding (30 %), cardiac (30 %), infections (22 %), and thrombosis (14 %).18 After 6 months readmissions decreased, but after 2 years bleeding admissions were more frequent. The factors associated with admissions after LVAD implant were preoperative anemia, higher levels of pro brain natriuretic peptide, lower glomerular filtration rate, and higher right atrial/pulmonary artery wedge pressure ratio (as a measure of impaired right ventricular function).18 A similar study by the Cleveland Clinic analyzed 118 HeartMate II LVAD patients and found that 52 % had 177 unplanned hospital readmissions: 87 were nondevice related, mainly from progression of underlying cardiac disease, and 90 were device related, largely due to device infection.19 More DT patients were readmitted, and overall 25 days were spent in hospital in the first 12 months.19 From a cost analysis perspective, patients with DT are estimated to live 4.4 years on average beyond the first year, but resource utilization by LVAD patients after rehospitalizations is large.20 A strategy to reduce hospitalizations is critical, as currently LVAD-DT does not meet the cost-effective benchmark.20,21 Unplanned hospitalizations are common, and increase with increasing time on mechanical support. The avoidance of hospitalization requires a multidisciplinary team of specialists that can aid patients and caregivers in identifying a good support system and home environment. Close attention needs to be given to patients with significant comorbidities (i.e. diabetes, renal dysfunction, peripheral vascular disease) and longer hospital stay post implant.22 Different strategies to reduce rehospitalization may include: follow up in the post-operative period, with weekly visits determined by the proximity to the surgical procedure; assigning coordinators to followup on tests after the patient has been discharged; and partnering with community hospitals or physicians who understand ventricular assist device technology and management. A recent occurrence of a devicerelated complication and the general status of the patient aids early recognition of potential complications and also provides an opportunity for further educating the patient and his or her family about device function and alarm recognition.
Emerging Applications
Developments in technology, patient selection and other therapies used in conjunction with mechanical circulatory support are being used to reduce the risks associated with and improve the outcomes of LVAD therapy. There is a focus on improving the hemo-compatibility of devices to reduce thrombotic and bleeding events; developing software algorithms that allow pulsatility parallel to a patient’s functional needs; the creation of smaller devices for less invasive procedures and shorter recovery; and the development of totally implantable designs that use wireless energy transfer systems.
Pulsatility
Recent research has produced insights into vascular responses to pulse pressure. It has been found that pulse amplitude is related to the endothelial production of nitric oxide and vasodilation, and that pulse pressure improves circulation in the capillary beds of end organs.23 Moreover, significant hemodynamic benefits of PF-LVADs can be seen in total cardiac output and lower pulmonary pressures and left atrial pressure, providing superior unloading compared to CF-LVADs. A return to pulsatility may reduce the number of adverse events, as studies have shown that patients with low pulsatility have increased non-surgical bleeding episodes.24 Observational studies have demonstrated that PF-LVADs may be better at inducing myocardial recovery than CF-LVADs, possibly by enhancing coronary flow, as noted by improvements in systolic and diastolic function, and a reduction in brain natriuretic peptide and extracellular markers.25 Based on these observations, there is now interest in developing algorithms to generate pulse pressure in an attempt to reduce adverse events associated with CF-LVADs.
The new Thoratec HeartMate 3 model is currently undergoing a clinical trial (MOMENTUM 3). The new pump design provides centrifugal flow and improves hemo-compatibility by having large gaps between the impeller and the housing that reduce red blood cell destruction. It employs a fully magnetic levitated rotor that can provide flows between 2.5 and 10 L/min and produces a near-physiological pulse pressure of 25 mmHg every 2 seconds.
Miniaturization
Another important aspect of device technology is reducing its size to allow shorter implant times, less invasive surgery and the potential expansion to other AHF populations. HeartWare MVAD is a pump with an axial impeller that uses hydrodynamic and magnetic force to move the rotor. The flow path exits the device perpendicular to the rotor’s orientation and uses modifiable pulsatility patterns, which may reduce arteriovenous malformation.
Partial Support and Myocardial Recovery
Reports of functional recovery following LVAD implantation have accelerated research in the field of myocardial regeneration. The rates of sustained cardiac recovery across the studies range from 1 to 36 %, with most patients who demonstrate substantial improvement in left ventricular ejection fraction having a non-ischemic dilated cardiomyopathy as the etiology of their heart failure, younger age and shorter disease duration.26 Initial reports from observational trials have noted that continued use of neurohormonal blockade might promote recovery. A prospective study of 20 LVAD patients receiving maximal doses of beta-blocker, angiotensin-converting-enzyme inhibitor, spironolactone and digoxin followed by high-dose clenbuterol reported complete normalization of left ventricular size and function in 12 patients who eventually underwent device explantation.27 Of these individuals, the estimated survival without recurrence of heart failure was 83 % at 1 and 3 years.27 Though these findings have yet to be reproduced in larger trials, national registries report 1–5 % explantation rates.7 These low rates may be explained by variations in medical therapy added to LVAD, variable unloading protocols and a mixture of populations by type and duration of heart failure.28 Mechanical unloading does change the myocardial structure and function as early as 30 days post implantation.29 Despite the structural changes seen after mechanical support, a gap still exists between the positive morphological changes seen and complete recovery in function. This may be due to the persistence of sarcomere contractile dysfunction after implantation.30
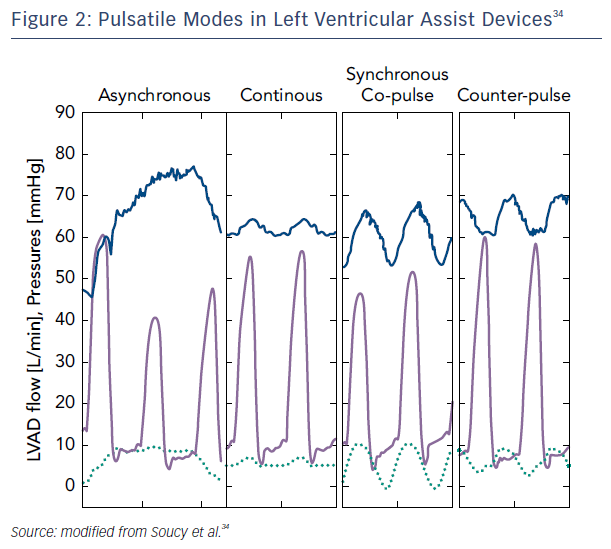
A more tangible approach for recovery may be to partially support the left ventricle by rotational speed modulation when synchronized with the cardiac cycle. Studies of patients who were partially unloaded (lower speeds) have shown improvement in myocardial performance, including increased peak oxygen consumption, resting myocardial blood flow, and lower myocardial oxygen consumption.31 This mode of operation on newer devices with the addition of pulsatility may create the right environment for cardiac remodeling and potential recovery. Indeed, synchronous unloading of the left ventricle by the assist device can be adjusted to deliver maximum flow either during systole (co-pulsation, which increases the speed during systole and decreases speed in diastole) or diastole (counter-pulsation, which increases the speed during diastole and decreases speed during systole). The former generates an arterial pulse, while the latter unloads more effectively while enhancing coronary flow and end-organ perfusion.32,33 Asynchronous mode, independent of the native heart rate, has the advantage of not requiring a trigger and combining intermittent co-pulsation and counter-pulsation support.34
Transcutaneous Energy Transmission System
This type of system has been in development since the 1960s and has limited applications. The use of this technology can potentially reduce driveline infections, which is one of the main causes of rehospitalization. The technology transfers power from an external coil to a subcutaneouslyplaced internal coil by using magnetic fields. One caveat is the misalignment during charge and risk of heat production.35 More recently, the freerange resonant electrical energy delivery (FREE-D) system has used coil mechanics. Its benefit over the older system is it is able to transfer power wirelessly across distances of a meter.36 The potential application of a total implantable system will allow patients to become more comfortable with their device, as being “wireless” may boost their mobility and autonomy, and positively impact their quality of life.36
End of Life after LVAD
While ventricular assist devices help provide significant symptom relief for patients, patients continue to have many other symptoms that persist including physical pain, major depression, and organic mental syndromes. As such, extensive discussion with patients and their caregivers is required prior to LVAD implantation so they have a realistic sense of what life is like with a LVAD as well as having the chance to express their wishes.
Recently the Center for Medicare Services has required that a palliative care physician be part of the core LVAD team. Ideally the palliative care physician should meet with the potential patient prior to LVAD to clarify the goals of care. The palliative care team can assist with pain and symptom management postoperatively, and with transition to end-of-life care when appropriate.
Living with a LVAD requires extensive commitment from the primary caregiver. Persons defined as primary caregivers include spouses, children, or even close friends. A primary caregiver who is willing to commit to indefinite 24-hour support of the patient after LVAD implantation needs to be identified and educated regarding the extent and nature of the commitment before LVAD placement. Further study is required to define the degree of caregiver burden among this patient population and effective strategies to reduce this burden.
Conclusion
The heart failure population is expected to increase to >8 million people in the US by 2030, and heart failure is likely to become the number one cause of disability in the country.37 The total cost of management will increase exponentially, and thus strategies to mitigate the disabling nature of the disease are required. LVADs are one treatment strategy and have advanced significantly since their early applications to become a viable option to manage AHF in the long term. It is hoped that improvements in circulatory systems, the miniaturization of devices, re-introduction of PF and the availability of a fully implantable system will improve the beneficial effects of LVAD therapy while limiting the complications associated with mechanical support. In the future, it is expected that strategies combining LVADs and pharmacological or cell-based therapy may ultimately lead to full myocardial recovery.