Acute coronary syndrome (ACS) occurs most often when a vulnerable intracoronary plaque ruptures or erodes to expose a prothrombotic core, precipitating thrombus formation by way of platelet activation,1–3 The spectrum of ACS includes non-ST segment-elevation (NSTE) ACS, where a vulnerable plaque threatens downstream coronary perfusion manifest as an increase in symptom frequency and/or duration (unstable angina), and includes biomarker-proven myocardial necrosis from an NSTE myocardial infarction. Transmyocardial injury from complete thrombotic occlusion of the coronary artery results in an ST-segment elevation myocardial infarction (STEMI).4,5
The spectrum of adverse outcomes in ACS includes ischemic complications of the index event, recurrent ischemic events after initial treatment, and complications from guideline-directed pharmaceutical and procedural interventions.
Patients identified as likely or definite ACS are typically managed following either an “early invasive” or “ischemia-guided” strategy. Patients in both groups receive similar upstream oral and parenteral medications, while patients following an early invasive strategy undergo coronary angiography and provisional percutaneous coronary intervention (PCI) based on immediately-available clinical, electrocardiogram, and laboratory data. Utilizing risk scores, and considering the patient’s clinical presentation, helps guide selection of the two strategies, where patients with a thrombolysis is myocardial infarction (TIMI) risk score ≥3 or a Global Registry of Acute Coronary Events score >140 benefit from an early invasive strategy.4–7
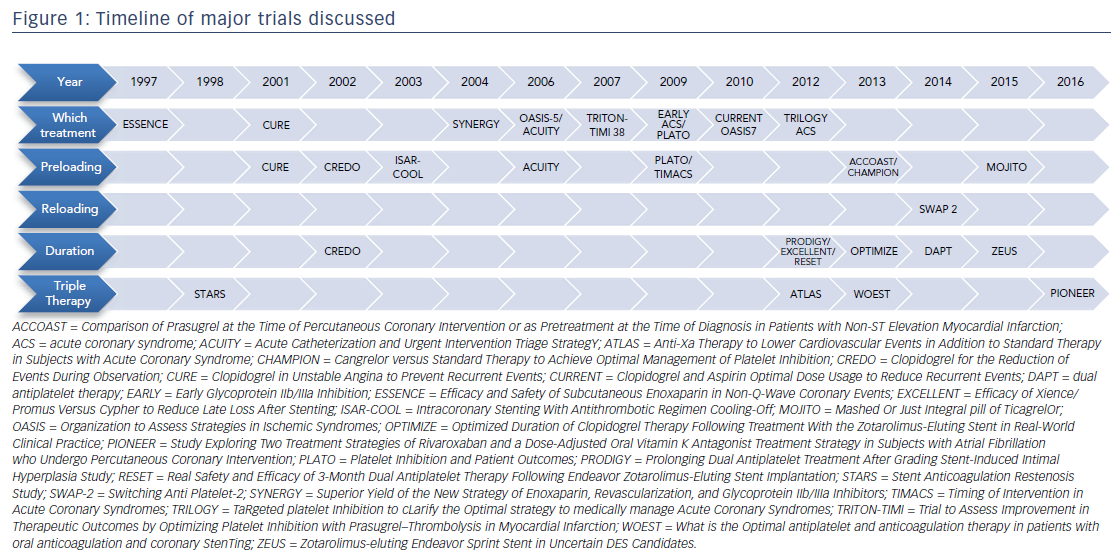
Central to the controversy in treating patients presenting with an ACS is balancing safety and efficacy, which poses challenges for the practicing clinician. For example, there are nearly 3 million combinations of oral medications at different durations in patients who have a concurrent need for anticoagulation.8 This review utilizes landmark studies to address five clinical questions central to the controversy of balancing safety and efficacy: (1) which treatment to give; (2) when to start treatment; (3) how to switch between oral medications; (4) how long to treat; and (5) how to manage antiplatelets in patients on systemic anticoagulation. A timeline of these landmark studies is provided in Figure 1.
Which Treatment to Give
Parenteral Anticoagulants
Consensus does not exist on the appropriate combination of medications. For example, the European Society of Cardiology (ESC) recommends against upstream use of a glycoprotein IIb/IIIa inhibitor prior to defining the coronary anatomy, whereas the American Heart Association (AHA)/ American College of Cardiology (ACC) suggests that it is reasonable to use prior to angiography in high-risk patients.9,10 Data support the use of unfractionated heparin (UFH), enoxaparin, and fondaparinux to reduce ischemic events in both ischemia-guided and early invasive strategies. Consideration can be given to using bivalirudin or adding a glycoprotein IIb/IIIa inhibitor to heparin in patients treated with an early invasive strategy. In clinical practice, UFH is most often selected initially because of its low cost, short half-life, ease of monitoring, lack of renal clearance, and ability to quickly reverse with protamine.
In the era before routine oral treatment with dual antiplatelet therapy (DAPT), a meta-analysis of the TIMI 11b and Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events trials demonstrated that enoxaparin reduced the risk of death or myocardial infarction by 20 % when compared to UFH. Part of the explanation of this benefit was that enoxaparin has a higher affinity for factor Xa than IIa compared to UFH, and therefore, greater inhibition of thrombin generation by interrupting the coagulation cascade closer to its root.11 It is important to note that most patients were treated with an ischemiaguided strategy, and fewer than 10 % of patients in the TIMI 11b trial underwent urgent revascularization within the first 8 days.11
The Superior Yield of the New Strategy of Enoxaparin, Revascularization, and Glycoprotein IIb/IIIa Inhibitors trial compared enoxaparin to UFH in 10,027 patients treated with an early invasive strategy, and found no difference in mortality between the groups.12 In this trial, nearly 60 % of patients were treated with a P2Y12 inhibitor and a glycoprotein IIb/ IIIa inhibitor. Investigators additionally found that patients who switched between arms had a higher rate of bleeding, which was thought to be due to the difficulty of dosing and the rapid monitoring of enoxaparin levels around the time of PCI because of its long half-life.12
In the Organization to Assess Strategies in Ischemic Syndromes (OASIS) 5 trial, 20,078 patients were randomized in a double-blind fashion to the factor Xa inhibitor fondaparinux or enoxaparin, with 60 % of the study population treated with an early invasive strategy and over 40 % treated with either PCI or coronary artery bypass grafting (CABG) while hospitalized. There was no difference in death or myocardial infarction at 9 days, but after 30 days, the fondaparinux group had a lower rate of bleeding (2.2 % versus 4.1 %) and death at 30 days (295 versus 352 patients).13 There was an increased incidence of catheter thromboses (0.9 % versus 0.3 %) among the patients who underwent coronary angiography, which raised concern about its use in patients undergoing a routine invasive strategy. The ESC recommends fondaparinux as the first-line anticoagulant agent in ischemia-guided strategies, whereas the AHA/ACC makes no preference.4,10
A meta-analysis of over 30,000 patients from six randomized, controlled trials undergoing an ischemia-guided strategy found that routine use of glycoprotein IIb/IIIa inhibitors reduced the odds of death or myocardial infarction by 9 % prior to PCI, particularly in patients at the highest ischemic risk.14 Patients in these trials were rarely treated upstream with DAPT, and as the practice of DAPT has increased, upfront IIb/IIIa inhibitors have become less popular.
The introduction the direct thrombin inhibitor bivalirudin further pushed IIb/IIIa inhibitors out of favor. The Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial demonstrated in nearly 14,000 patients with ACS that bivalirudin was not inferior to heparin and a IIb/IIIa inhibitor for ischemic outcomes at 30 days, with the benefit of reduced major bleeding events (3.0 % versus 5.7 %). In one study, 64 % of patients were pretreated with a P2Y12 inhibitor.15 The reduction in bleeding events had the strongest interaction with ACS patients who underwent PCI by femoral access.16
The Early Glycoprotein IIb/IIIa Inhibition ACS trial further supported avoiding routine, upfront eptifibatide in patients treated with clopidogrel (75 % of population), by demonstrating no added benefit of the IIb/IIIa inhibitor and an increased risk of bleeding.9
In a Bayesian analysis of 18 randomized, controlled trials comparing heparin to bivalirudin, the overall unadjusted mortality was not different between the groups. Patients treated with bivalirudin had fewer bleeding events, but a higher rate of early stent thrombosis. The bleeding advantage of bivalirudin was reduced when the results were stratified by transradial access, planned glycoprotein IIb/IIIa use with bivalirudin, or administering ticagrelor or prasugrel. Interestingly, the use of ticagrelor or prasugrel did not reduce the risk of stent thrombosis in patients randomized to bivalirudin.17
In current practice, parenteral anticoagulation for ACS is typically a decision between heparin and provisional IIb/IIIa inhibitors versus bivalirudin if proceeding to PCI.
Oral Antiplatelet Medications
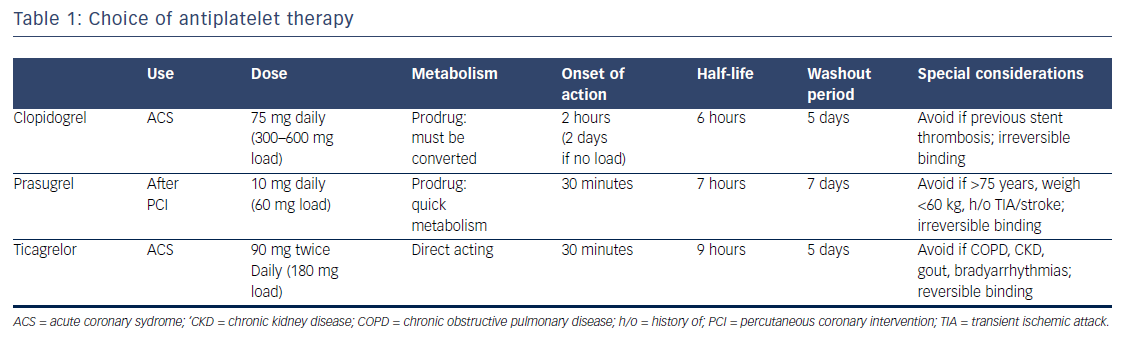
While its utility in the primary prevention of cardiovascular events is subject to debate, in patients not on systemic anticoagulation, aspirin is accepted as the standard of care in the treatment of ACS.18 Oral P2Y12 inhibitors (clopidogrel, ticagrelor, and prasugrel) now serve as mandatory support to protect against stent thrombosis in ACS, as well as to reduce all-cause ischemic events post-ACS. Guidelines recommend 1 year of DAPT, regardless of whether a stent is placed in a patient with NSTE-ACS.4 Clopidogrel and prasugrel are thienopyridines that irreversibly block the adenosine diphosphate (ADP) binding site on the P2Y12 receptor and inhibit platelet aggregation. Ticagrelor is a cyclopentyl-triazolo-pyrimidine agent that binds directly on the P2Y12 receptor (apart from the ADP binding site) without enzymatic conversion, and likewise, inhibits ADPinduced platelet aggregation.19,20 The characteristics of these antiplatelets are represented in Table 1.
Evidence supporting the use of DAPT in ACS stems from the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) trial, which demonstrated the benefit of adding clopidogrel with a loading dose of 300 mg to aspirin in ACS, regardless of whether PCI was pursued.21 A 600 mg dose of clopidogrel at least 2 hours prior to PCI is further associated with improved platelet inhibition compared to 300 mg.22
The Clopidogrel and Aspirin Optimal Dose Usage to Reduce Recurrent Events–OASIS 7 demonstrated that loading with 600 mg of clopidogrel followed by 150 mg daily on days 2–7, compared to 300 mg followed by 75 mg, reduced the risk of stent thrombosis by 32 % at the expense of a 24 % increase in major bleeds among patients undergoing PCI for ACS.23 While stent thrombosis risk was reduced, there was no difference in the primary outcome of cardiovascular death, myocardial infarction, or stroke at 30 days between the clopidogrel high- and low-dose arms.24 This trial also demonstrated that, after an initial load of >300 mg aspirin, a low dose is equivalent to continued high dose on days 2–30.23
Ticagrelor was compared to clopidogrel in the PLATelet inhibition and patient Outcomes (PLATO) trial, which demonstrated a reduced rate of death, myocardial infarction, or stroke, without an increase in the rate of overall major bleeding, but at the expense of a slightly higher risk of nonprocedure- related bleeding. This was true for patients undergoing both ischemia-guided and early invasive treatment strategies. Ticagrelor also conferred a 33 % relative risk reduction in stent thrombosis compared with clopidogrel.25,26 Regional variability with improved performance of clopidogrel (and no benefit of ticagrelor) in North America was seen in the trial, perhaps related to the increased use of high-dose aspirin in the US.27 As a result, the Food and Drug Administration (FDA) recommends <100 mg aspirin given concurrently with ticagrelor.
Prasugrel reduced the rates of ischemic events, including stent thrombosis, in patients undergoing PCI for ACS compared with clopidogrel at the expense of increased major bleeding, including fatal bleeding in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel (TRITON)-TIMI 38 trial.25 In the trial, 55 % of patients were treated with a glycoprotein IIb/IIIa inhibitor, and 3 % with bivalirudin, compared with 26 % and 2 %, respectively, in the PLATO trial. Other differences between these trials include clopidogrel loading dose (600 mg in the PLATO trial and 300 mg in the TRITON trial) and timing of drug loading relative to PCI (4 hours on average before PCI in the PLATO trial and at the time of PCI or immediately post in the TRITON trial). In a follow-up study, investigators found a greater inhibition of platelet aggregation measured in vitro with a 60 mg loading dose of prasugrel compared to a 600 mg loading dose of clopidogrel.28 Of note, no benefit or potential harm was seen among patients treated with prasugrel who were older than 75 years, weighed less than 60 kg, or who had a prior cerebrovascular accident.29 A black-box warning advises against use in these groups. Prasugrel is also superior to clopidogrel in preventing stent thrombosis.30 In patients with ACS who do not undergo revascularization, treatment with prasugrel versus clopidogrel resulted in similar rates of death from cardiovascular causes, myocardial infarction, or stroke in patients under the age of 75 years in the TaRgeted platelet Inhibition to cLarify the Optimal strategy to medically manage Acute Coronary Syndromes ACS Trial.31
In summary, current guidelines agree with a loading dose of aspirin at THE time of presentation, and a maintenance low dose in patients after ACS. Treatment with dual antiplatelet therapy is recommend for patients with ACS, regardless of treatment strategy, but prasugrel is approved only among patients undergoing PCI.4,5 The timing of initiation of the P2Y12 inhibitor in the management of ACS remains a subject of much debate, and is discussed next.
When to Start a Second Antiplatelet Agent in NSTE-ACS: Preloading
Despite solid agreement in the guidelines, and support for preloading from both the ESC and AHA/ACC, resistance to preloading with either clopidogrel or ticagrelor persists.10,32,33 Frequently-cited concerns about preloading with P2Y12 inhibitors are the need to delay CABG in ACS patients with surgical disease and/or increased CABG-related bleeding. However, the need for in-hospital CABG in patients presenting with ACS ranged from 4.5 % to 11.1 % in the CURE, ACUITY, and PLATO trials. While it occurs in a minority of patients, there is a wide range of CABG-related major bleeding, which is reported to occur 7.0–52.9 % of the time.25,34,35 Conversely, there is also fear that administration of the oral medications might not be metabolized effectively, and treatment could be ineffective in the period shortly after oral administration.36 This controversy drives at the crux of balancing safety and efficacy.
Early data from the CURE trial demonstrated the benefit of early antiplatelet treatment with P2Y12 inhibitors, where a dose of clopidogrel reduced the risk of the primary combined endpoint of death, myocardial infarction, or stroke (PCI median was 6 days). With clopidogrel pretreatment, the event curves diverged early and prior to PCI.21 Furthermore, in the ACUITY trial of bivalirudin versus heparin and a glycoprotein IIb/IIIa, 68 % of patients were pretreated with a thienopyridine prior to PCI, and had a trend toward fewer ischemic events compared to those not pretreated.16 In the PLATO trial, all patients were preloaded with ticagrelor, and while the benefit occurred when treatment was initiated prior to PCI, the differences in timing of initiation were not analyzed.25 In a meta-analysis of patients undergoing an early invasive strategy, clopidogrel pretreatment was not associated with a reduction of death nor increased risk of major bleeding, but did show a decreased rate of major coronary events or myocardial infarctions.37
There is overall less benefit to pretreatment when there is a shorter time to angiography, likely due to delayed transit and absorption of the swallowed tablets.36 The Mashed Or Just Integral pill of TicagrelOr trial circumvents this concern, and demonstrated improved function (reduced platelet reactivity) in STEMI patients within 1 hour of administering crushed ticagrelor, suggesting a pharmacokinetic advantage over whole tablets.38
Direct evidence showing that delaying angiography for preloading P2Y12 inhibitors is lacking. The hypothesis generated from evidence based on the Clopidogrel for the Reduction of Events During Observation (CREDO) trial was that pretreatment with 300 mg clopidogrel 3–24 hours prior to PCI reduced event rates, but only in the subgroup of patients treated at least 6 hours prior to PCI.39 However, the Intracoronary Stenting with Antithrombotic Regimen Cooling-Off trial found that pretreatment with antithrombotic therapy, including clopidogrel, for 3–5 days increased mortality compared with early (<6 hours) angiography (Tirofiban was used in all patients).40 In contrast, the Timing of Intervention in Acute Coronary Syndromes trial compared early (<24 hours) versus delayed (>3 days) coronary angiography, and found no overall difference in the primary endpoint of stroke, myocardial infarction, or death at 30 days; however, the majority of patients (>90 %) were treated with a loading dose of clopidogrel prior to PCI.7
Despite questions raised by these trials, there has not been a prospective, randomized trial examining optimal timing of clopidogrel or ticagrelor loading.41 The Comparison of Prasugrel at the Time of Percutaneous Coronary Intervention or as Pretreatment at the Time of Diagnosis in Patients with Non-ST Elevation Myocardial Infarction trial randomized patients to pretreatment with prasugrel (30 mg prior to PCI and 30 mg after) versus loading after PCI. The trial was stopped early, given no benefit of the pretreatment arm, and increased bleeding events.42 These randomized data have tempered enthusiasm for preloading.33
Patients undergoing PCI who have not been pretreated with an oral P2Y12 inhibitor are often given IIb/IIIa inhibitors during the PCI, following data demonstrating a reduction in ischemic events in these patients.43 Cangrelor is a recently FDA-approved parenteral P2Y12 inhibitor that offers rapid platelet inhibition.5 When started a median of 4.4 hours before PCI in a pooled analysis of nearly 25,000 patients, cangrelor reduced the combined endpoint of death, myocardial infarction, revascularization, and stent thrombosis (3.8 % versus 4.7 %), without an increase in Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries severe/life-threatening bleeding, but with an increase in TIMI mild and ACUITY major bleeding. A majority of patients were given a loading dose of clopidogrel immediately prior to PCI, with 75 % of patients treated with unfractionated heparin, and 25 % with bivalirudin.44
A reasonable strategy for the practicing cardiologist involves loading patients presenting with ACS with a P2Y12 inhibitor, and utilizing radial access and a short course of IIb/IIIa inhibitors (3 hours, not 12–24 hours), if needed, to minimize bleeding and maximize efficacy.
How to Switch Oral Medications: Reloading
After initiating treatment with an oral P2Y12 inhibitor, often patients need to switch to an alternative medicine due to insurance coverage or adverse events, including upper gastrointestinal bleeding with prasugrel or dyspnea with ticagrelor.29,41 Due to differences in kinetics and the mechanism of action, switching medicines is not as simple as starting one and stopping another. Guidelines do not currently support a method of switching, and clinical outcome data are sparse.
Both prasugrel and clopidogrel irreversibly bind to the ADP receptor binding site on the platelet. Clopidogrel has a delayed effect for full platelet inhibition, and along with prasugrel, must be metabolically activated (Table 1).19,39 Ticagrelor binds reversibly and independently from ADP outcompetes binding with prasugrel or clopidogrel.19 Due to its 9-hour half-life and reversible binding, platelet function recovers quickly with the cessation of ticagrelor. Unless a loading dose is given to allow continued exposure, prasugrel and clopidogrel are only transient in the circulation, and new platelets remain activated (since no longer bound by ticagrelor), as they are released into the circulation. The Switching Anti Platelet-2 trial demonstrated the return of platelet reactivity when switching from ticagrelor to prasugrel, which was reduced, but not fully resolved with a reloading of prasugrel.45
While clinical outcomes are unknown, and anecdotal reports of adverse patient events abound, when an alternative antiplatelet is required, it is reasonable to reload with either clopidogrel or prasugrel when switching from ticagrelor. Alternatively, initiating ticagrelor or switching between clopidogrel or prasugrel does not require reloading.2,19,28 If a patient requires increased efficacy in their antiplatelet regimen, reloading with prasugrel or ticagrelor from clopidogrel is reasonable to strengthen the antiplatelet effect. Another consideration discussed below is that if patients require the initiation of anticoagulation due to atrial fibrillation, it is generally advised to switch to clopidogrel from a more potent P2Y12 inhibitor to limit bleeding.41
Treatment Duration
While guidelines have long supported 1 month of DAPT for bare-metal stents, and 12 months for drug-eluting stents, a decade of trials has pushed the acceptable duration of dual antiplatelet therapy in both directions, based on the patient’s clinical condition, complicating a universal approach to therapy. A recent update to the AHA/ACC guidelines now recommends a minimum of 6 months of dual antiplatelet therapy following the placement of a drug-eluting stent, but practice variability exists.46
The threat of late stent thrombosis (occurring between 1–12 months) with the discontinuation of dual antiplatelet therapy drives prolonged treatment.47 Early real-world reports with sirolimus and paclitaxel-eluting stents demonstrated a 1.3 % risk of stent thrombosis in three hospitals and a 45 % mortality. Early discontinuation of antiplatelet therapy emerged as a major predictor of stent thrombosis.48 The CREDO trial emphasized the importance of 12 months versus 1 month of clopidogrel treatment after elective bare-metal stent implantation, with a 26.9 % relative risk reduction of the combined endpoint of death, myocardial infarction, or stroke.39
The DAPT trial tested continuing dual antiplatelet therapy (65 % clopidogrel, 35 % prasugrel) beyond 12 months after coronary stenting, and demonstrated reduced ischemic endpoints, including stent thrombosis and major adverse cardiovascular and cerebral events.49 Additional benefits included a reduction in myocardial infarction. More than half of the total benefit was unrelated to stent thrombosis, reaffirming the role of DAPT in reducing non-target vessel ischemic events. These benefits, however, came at a 1 % absolute increase in major bleeding events. A bleeding risk calculator to assist in clinical decision-making was derived and validated, with scores above 2 associated with a greater benefit of continuing dual antiplatelet therapy beyond 12 months.50
Numerous trials demonstrate non-inferiority for the shorter duration of dual antiplatelet therapy in specific scenarios. The Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study trial tested 6 months versus 24 months of clopidogrel in patients treated with a mix of bare-metal and drug-eluting stents, and found no difference in the risk of death, myocardial infarction, or cerebrovascular accident, but an increased risk of hemorrhage in the 24-month clopidogrel group.51 The Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting trial demonstrated that 6-month dual antiplatelet therapy did not increase the risk of target vessel failure at 12 months compared with a 12-month dual antiplatelet strategy. This trial has been criticized because of the wide non-inferiority margin that some consider clinically unaceptable.52
The Zotarolimus-eluting Endeavor Sprint Stent in Uncertain DES Candidates trial demonstrated superiority of 1 month of dual antiplatelet therapy in patients receiving a zotarolimus-eluting stent with a fast-release profile versus a bare-metal stent in reducing myocardial infarction and target vessel revascularization.53 Utilizing the same Endeavor zotarolimus-eluting stent, the Real Safety and Efficacy of 3-Month Dual Antiplatelet Therapy Following Endeavor Zotarolimus-Eluting Stent Implantation trial demonstrated noninferiority of a 3-month dual antiplatelet strategy versus 12 months, without an excess risk of stent thrombosis after cessation of clopidogrel in the 3-month group.54 In addition, the Optimized Duration of Clopidogrel Therapy Following Treatment With the Zotarolimus-Eluting Stent in Real-World Clinical Practice trial demonstrated non-inferiority of 3 months versus 12 months of DAPT in stable, low-risk patients treated with a zotarolimus-eluting stent.55
When considering the duration of dual antiplatelet therapy, balancing the risk of bleeding and benefit of preventing ischemia is difficult and calls for the development of decision tools, such as the DAPT score, to help guide individual treatment deicsions.50
How to Address Triple Therapy
Over 5 % of patients referred for PCI have concurrent atrial fibrillation. Patients treated with dual antiplatelet therapy, as well as anticoagulation (so-called “triple therapy”), have an excess risk of major bleeding beyond 10 % per year,56,57 While there is guidance from multiple groups on the management of these patients, until recently, there have been little randomized, controlled data.58–60
Warfarin is effective post-ACS at decreasing recurrent myocardial infarction from a meta-analysis of nearly 6,000 patients treated without stents, but at an increased rate of bleeding.61 For preventing ischemic complications following stent placement, DAPT with ticlopidine is superior to aspirin and warfarin, based on the Stent Anticoagulation Restenosis Study trial.62 The Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome ACS-TIMI 51 trial demonstrated secondary risk reduction with a low-dose rivaroxaban treatment shortly after ACS. This highlights the role of factor Xa in thrombosis in patients with ACS, and the benefits of Xa inhibition, but also the risk of bleeding. While triple therapy is often inevitable, practice has centered around limiting the duration of treatment with triple therapy by either using bare-metal stents or balancing the risk of stroke and stent thrombosis and lowering the international normalized ratio goals to 2.0–2.5.57
The What is the Optimal antiplatelet and anticoagulation therapy in patients with oral anticoagulation and coronary StenTing trial tested the innovative idea of dropping aspirin from the triple-therapy cocktail in patients with atrial fibrillation and a stent. The major finding was a 64 % relative risk reduction in any bleeding event (not just major bleeding) at 1 year in patients treated with a vitamin K antagonist and clopidogrel versus triple therapy.63 While the trial was small (573 patients), there was no evidence of increased thrombotic risk of dropping aspirin.
Recent data from the Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Intervention (PIONEER) trial demonstrated the improved safety of dropping aspirin in favor of low-dose rivaroxaban (15 mg daily) in addition to a P2Y12 inhibitor versus standard triple therapy.64 Randomization of the 2,124 patients occurred in a 1:1:1 ratio, and included a group of patients also treated with very low-dose rivaroxaban (2.5 mg twice daily). In the group of patients treated with 15 mg rivaroxaban and a P2Y12 inhibitor, there was a 41 % relative risk reduction of clinically-significant bleeding. Likewise, there was a 37 % relative risk reduction when comparing 2.5 mg rivaroxaban twice daily with triple therapy with warfarin. Patients were excluded if they had a recent gastrointestinal bleed, anemia of unknown cause, recent transient ischemic attack or stroke, or if the creatinine clearance was <30 mL/min.64 In addition to the PIONEER trial, three other trials assessing other oral anticoagulants are ongoing to help further elucidate the question of minimizing both bleeding and thrombotic risks in these patients.65 These trials are designed to assess safety. Demonstration of non-inferiority or superiority for bleeding will be expected, given the high risk of bleeding with triple therapy and the relative low risk of embolic events. Proving efficacy is limited by the large number of patients required, and therefore, the efficacy of dropping aspirin will likely remain an area of controversy.
Conclusion
Patients undergoing coronary stenting have numerous medical therapies to help minimize adverse events, including stent thrombosis, while balancing the risk of bleeding. The practicing cardiologist faces numerous uncertainties as they treat patients with ACS. This review explored the controversies, including the choice initial antiplatelet and anticoagulant combination, decision of when to load with a second P2Y12 inhibitor and how to transition between them, how long to treat after a stent, and how to manage patients on oral anticoagulants.
Navigating the different treatment possibilities remains a daunting task. Within a vast knowledge base and excellent trials, controversy still exists in how to best manage patients presenting with ACS. While guidelines and assistance exist, ultimately many of these questions are best answered when considering the totality of the patient’s presentation. Tailoring the therapy for each patient combines the art and science of clinical medicine, with the emphasis on the individual patient.