Transcatheter aortic valve replacement (TAVR) has been established as a therapeutic option for patients with severe symptomatic aortic stenosis who are considered to be of intermediate, high or prohibitive surgical risk.1–5 As a result of favorable TAVR outcomes and substantial improvements in transcatheter heart valve (THV) technologies and implantation techniques, the feasibility of broadening applications to the low-risk population is being evaluated.
Despite periprocedural complications being reduced with newer THV generations, the occurrence of conduction abnormalities and the need for permanent pacemaker implantation (PPI) remain the most frequent complications.6,7 Rates of PPI have not been significantly reduced but rather, with some technologies, have increased. 8 The long-term implications of PPI in the TAVR patient population remain unclear, and applicability in low-risk patients is a further consideration. In addition, short-term implications may jeopardize the minimalist TAVR approach, with increased use of electrophysiological studies and continuous EKG monitoring devices (i.e. Holter monitors, event monitors, or implantable loop recorders), and subsequent prolonged length of hospital stay.9–12
This article highlights conduction abnormalities after TAVR with a focus on basic conduction system anatomy in relation to the aortic valve, the mechanism, incidence, predisposing factors for occurrence, impact on mortality, and finally, proposed treatment algorithms for management.
Anatomy of the Conduction System
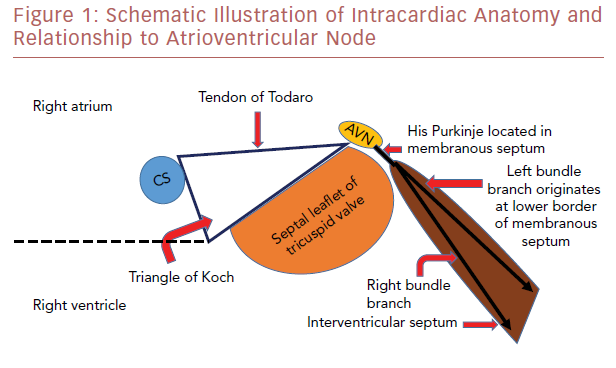
The atrioventricular node (AVN) is located within the triangle of Koch, which is demarcated by the tendon of Todaro, the septal leaflet attachment of the tricuspid valve, and the orifice of the coronary sinus (Figure 1). The AVN continues as the His bundle, tracking through the septum leftward to the central fibrous body. The central fibrous body is the area within the heart where the membranous septum (MS), the atrioventricular valves, and the aortic valve join in continuity. The left bundle branch exits within this area between the non-coronary cusp (NCC) and right coronary cusp (RCC) leaflets and travels along the septal surface of the left ventricular septum.13,14 The close relationship of the AVN and left bundle branch to the subaortic region explains the potential conduction abnormalities after percutaneous THV insertion.
Conduction Abnormalities after Surgical Aortic Valve Replacement
The most common conduction abnormality after surgical aortic valve replacement (SAVR) is left bundle branch block (LBBB). The incidence of new LBBB after SAVR has been reported to range from 6% to 32%.15–17 It is caused by injury to the conduction system at the interleaflet triangle of the NCC/RCC leaflets from direct surgical trauma during decalcification, mechanical compression, hemorrhage, or ischemia. 15–17 New LBBB after SAVR was found to be associated with worse 1-year survival, when compared with cases where LBBB did not develop. 15,18
SAVR can be performed by using either stented or stentless biological prostheses. Stented biological prostheses can be implanted in a supra-annular or intra-annular position; the valve does not generate a radial force that compresses the conduction system if implanted in a supra-annular position. Stentless valve prostheses are designed to achieve a more physiological flow pattern and superior hemodynamics in comparison with stented valves.19 Some generations of stentless valves require only one suture line to secure the valve. 19
Recent technological developments have led to an alternative, minimally-invasive option that avoids the placement of sutures, known as sutureless or rapid-deployment aortic valves (Su-AVR). Su-AVR, which combines features of both SAVR and TAVR, requires removal/decalcification of native leaflets, but depends on its intra-annular stent design with oversizing to anchor the prosthesis. 20 Conduction abnormalities associated with this valve type are more similar to with TAVR than SAVR.21
Conduction Abnormalities After Transcatheter Aortic Valve Replacement
TAVR prostheses are placed in an intra-annular position in closer proximity to the AVN and left bundle branch. In contrast to surgical valves, they are anchored into the aortic annulus and their stent frames generate a radial force expansion that may compress the conduction system.21 Slight oversizing is necessary in implant technique to secure the THV and reduce paravalvular regurgitation; however, excessive oversizing can result in increased compression of the conduction system.22 Overall, TAVR patients have a higher incidence of conduction abnormalities than patients who have conventional SAVR. 21
Incidence of New-onset Left Bundle Branch Block and Permanent Pacemaker Insertion
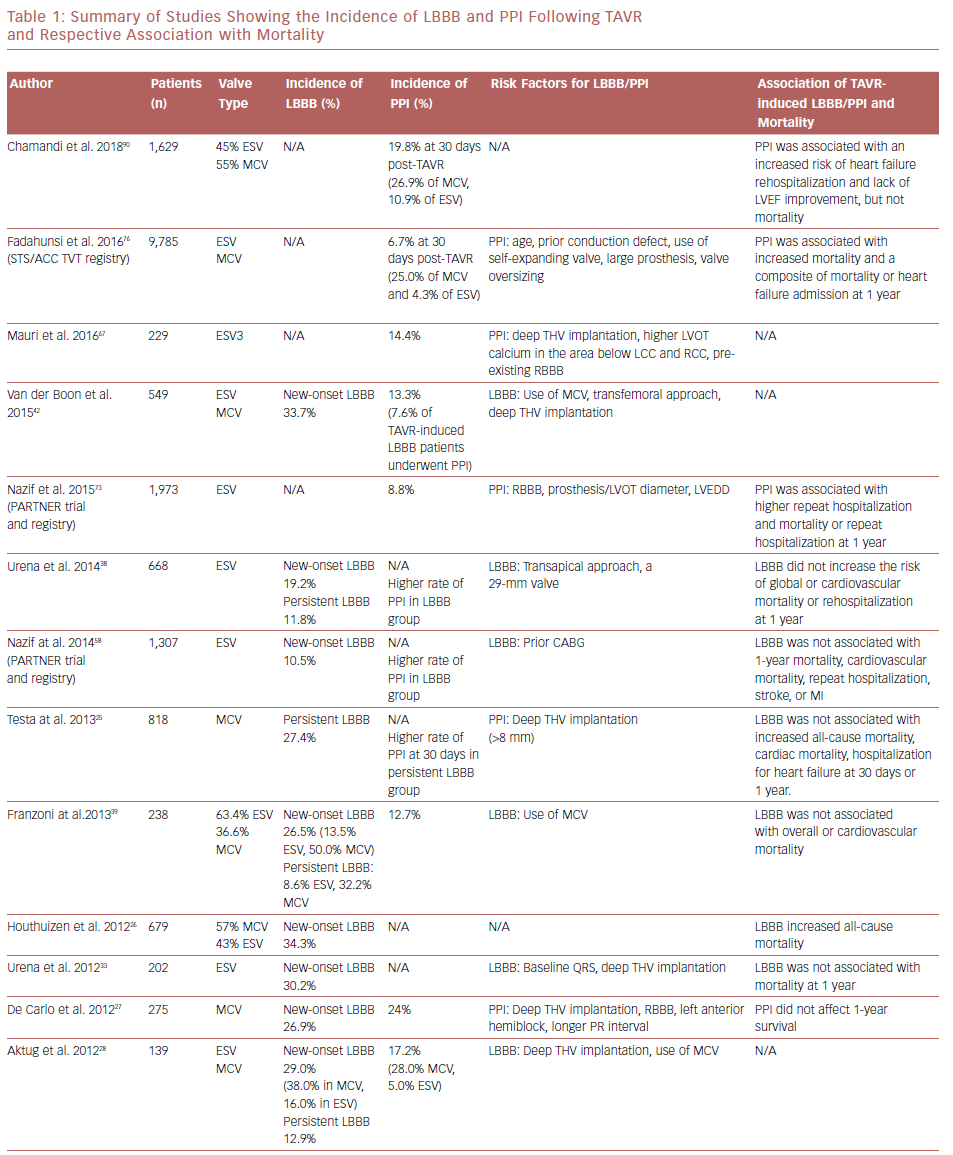
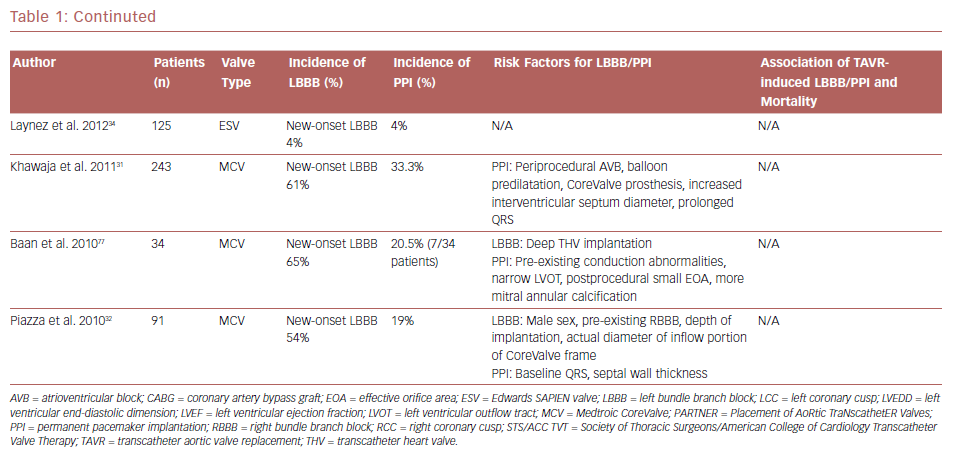
New-onset LBBB is the most frequent complication after TAVR.6 The incidence of new-onset LBBB ranges from 4% to 57%, with the rate of PPI ranging from 2% to 51%.23,24 The incidence of both new-onset LBBB and PPI are higher after implantation with the self-expanding CoreValve® system (MCV, Medtronic) than with the balloon-expandable SAPIEN or SAPIEN XT systems (ESV, Edwards Lifesciences); new-onset LBBB and PPI are 35–65% and 28%, and 3–30% and 6%, for MCV and ESV, respectively.25–34 Table 1 summarizes studies with their associated LBBB and PPI rates. The higher incidence of PPI in the MCV compared with the ESV has been confirmed in a randomized controlled trial.35
Overall, LBBB leads to an increased likelihood of new PPI early after TAVR. 36 However, one-fifth to nearly half of new-onset LBBB is temporary.37 Testa et al. studied 1,060 patients treated with MCV; 43.0% developed LBBB after TAVR, and this figure decreased to 27.3% at discharge and remained stable at 30 days.25 Urena et al. reported the rate of new-onset LBBB to be approximately 20.0% after TAVR with ESV and that 50.0% of new-onset LBBB resolved within a few days after TAVR, leading to a rate of new-onset persistent LBBB of approximately 10.0%.38 In another study, Franzoni et al. showed a higher incidence of LBBB following MCV (50.0%) than ESV (13.5%), which reduced by discharge to 32.2% for MCV and 8.6% for ESV, respectively.39
LBBB is also a predictor of late PPI after hospital discharge.40,41 In a recent meta-analysis, a higher rate of PPI at 1-year follow up was observed among patients with new-onset LBBB, compared with those who did not develop LBBB.41 The frequency of LBBB after TAVR has decreased significantly over time, especially with MCV THVs. This has been largely attributed to operator experience and the subsequent reduction in implantation depth.42 Nevertheless, the incidence of PPI has remained unchanged over time and has not been affected by operator experience.42 When interrogation of permanent pacemakers are performed, approximately 50% of patients are continuously paced, 25% are intermittently paced, and 25% have adequate atrioventricular conduction without the necessity of pacing. 37 The patient population with persistent LBBB who require PPI and have identifiable need upon follow-up interrogation still require improved understanding.
Newest Third-generation Transcatheter Heart Valves
Increased rates of PPI, ranging from 12.4% to 25.5%, have been reported with the use of the newest third-generation ESV SAPIEN 3, when compared with previous generations.43–49 This finding has been attributed to the incorporation of an external fabric cuff in the inferior part of the valve, intended to minimize paravalvular leak. Moreover, different stent expansion patterns of the SAPIEN 3 compared with the SAPIEN XT may play a role.50 In SAPIEN XT, the expansion area increased from the inflow level, reaching its peak at the outflow level; in contrast, the SAPIEN 3 has its largest expansion at the left ventricular outflow tract (LVOT) end, causing elevated localized pressure within the LVOT and thus higher rates of atrioventricular conduction disturbances.50 A higher (>70% aortic extension) valve depth implantation of this newest-generation THV may decrease PPI risk.43,44 Also, the next-generation self-expanding MCV Evolut Pro has been designed with an external pericardial wrap with the intention of reducing paravalvular leak. Early PPI rates in the first 60 patients were reported at 30 days at 11.7%.51 Although 6-month data suggest no significant change in PPI, data for this THV are limited.
Impact of Transcatheter Aortic Valve Replacement-induced Left Bundle Branch Block on Mortality
LBBB has been associated with increased morbidity and mortality in a broad population of patients, from healthy individuals to patients who have had MI and have established heart failure. 52,53 However, there are conflicting data about the impact of new-onset LBBB on mortality in post-TAVR patients. Several studies have failed to show the relationship between new-onset LBBB and mortality.25,27,32–34,38,54–58 In an analysis from the Placement of AoRtic TraNscathetER Valves (PARTNER) trial, persistent new-onset LBBB occurred in 10.5% of cases and was not associated with all-cause mortality, cardiovascular mortality, stroke, or MI. However, it was associated with a higher rate of repeat hospitalizations, PPI, and lack of improvement in left ventricular ejection fraction (LVEF).58 On the other hand, a large multicenter registry study by Houthuizen et al. reported that TAVR-induced LBBB is one of the strongest predictors of all-cause mortality in TAVR patients,26 and can neutralize the benefit of TAVR. A meta-analysis by Regueiro et al. confirmed a higher risk of cardiac death in patients with TAVR-induced LBBB after 1 year of follow up.41
Possible mechanisms of increased mortality for patients with TAVR-induced LBBB are progression to high-grade atrioventricular blocks (HAVB), and the development of dyssynchrony associated with the LBBB.26 LBBB causes left ventricular dyssynchrony, which has a similar effect to chronic right ventricular pacing and can lead to reduction in left ventricular function and remodeling. 59,60 Patients who develop left ventricular dysfunction from dyssynchrony are also susceptible to ventricular tachyarrhythmias, which could be another possible explanation for higher mortality in patients with TAVR-induced LBBB.26 One case report of a patient without pre-existing conduction abnormalities who died suddenly in the early phase after discharge, showed autopsy findings of a THV that had compressed the atrioventricular conduction system at the septum.61 Microscopic examination confirmed necrosis of the His bundle and left bundle branch as a result of mechanical compression, supporting progression to HAVB as a possible mechanism of sudden cardiac death (SCD).61
Advanced heart failure and SCD account for two-thirds of cardiac deaths in post-TAVR patients.62 LVEF ≤40% and new-onset persistent LBBB following TAVR were independently associated with an increased risk of SCD.62 Patients with new-onset persistent LBBB and QRS duration >160 ms had a greater SCD risk and most of them died within 6 months of TAVR.62 No increased risk of SCD was observed in patients with new-onset persistent LBBB and pacemaker implanted before hospital discharge, suggesting HAVB as the main cause of SCD in these patients. 62 The ongoing Ambulatory Electrocardiographic Monitoring for the Detection of High-Degree Atrio-Ventricular Block in Patients With New-onset PeRsistent Left Bundle Branch Block After Transcatheter Aortic Valve Implantation (MARE) study, with continuous EKG recording (up to 3 years) in patients with new-onset persistent LBBB following TAVR should provide more information on this issue.
Mechanism and Risk Factors of TAVR-induced LBBB, HAVB, and PPI
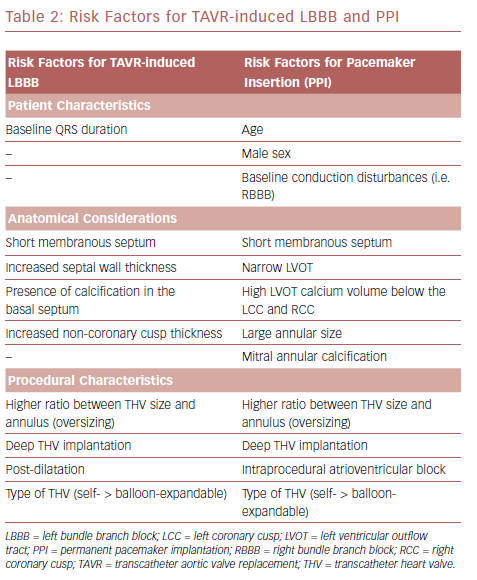
Atrioventricular conduction disorders and LBBB occur after both TAVR and SAVR as a result of the close proximity of the AVN and left bundle branch to the aortic valve.13 The His bundle is located between the MS and the posterior crest of the muscular septum; the lower end of MS is an anatomic landmark for the left ventricular exit point of the His bundle.13 Consequently, the MS length represents the distance between the aortic annulus and the His bundle. Hamdan et al. evaluated 73 patients with severe aortic stenosis who underwent contrast-enhanced CT before TAVR and found that MS length was the most powerful predictor of HAVB and PPI.63 Short MS, insufficient distance between MS length and implantation depth, and the presence of calcification in the basal septum facilitate mechanical compression of the conduction tissue by the TAVR prosthesis.63 On the other hand, a longer MS length may allow accommodation of more device penetration without causing conduction abnormalities.63
LBBB may develop before actual insertion of the valve device in >50% of cases. This can be caused by contact of the guidewire or compression of the LVOT by balloon dilatation.29,56 Patient- and procedure-related factors such as septal wall thickness, NCC thickness, depth of valve implantation within the LVOT, post-implantation dilatation, and large size and type of THV predicts LBBB or new conduction abnormalities after TAVR.38,54–56,64 Deep THV implants, greater than or equal to 6 mm, are associated with increased conduction abnormalities and pacemaker rate. 39,65,66 Moreover, higher ratio between prosthesis valve size and the annulus, (that is, oversizing) in the MCV is considered to be a predictor of new LBBB.39
Mauri et al. identified risk factors for PPI following TAVR with a balloon-expandable (SAPIEN 3) THV to be a high LVOT calcium volume in the area below the left coronary cusp and RCC, pre-existing right bundle branch block (RBBB), and lower implantation depth (Table 2).67 Tarantini et al. described a relationship between implantation depth and PPI rate after SAPIEN 3 implantation and proposed an implantation technique aimed at a maximum LVOT extension of the stent frame of less than 8 mm, which would result in a ventricular portion of approximately 40.0% depending on prosthesis size.44 Subsequent studies showed that implantation techniques aimed at a ventricular portion of <30.0% and <25.5% were the best discriminatory thresholds for reduced PPI risk.43,67 Optimal implantation depths for MCV and ESV are between 3 mm and 6 mm and 80% aortic:20% ventricular, respectively.
The radial force from the THV must be sufficient to ensure valve anchoring, but not interfere with the AVN and disturb the electrical conduction system.68 Radial force produced by the MCV and ESV was studied by Tzamtzis et al.69 In self-expanding THVs, the radial force is dependent on the diameter of LVOT.69 However, the radial force in the balloon-expandable THVs is associated with a more complex mechanism that involves the geometric and material properties of the stent, of the balloon and of the host tissue, as well as the technical aspects of the balloon inflation procedure.69
Pre-existing Right Bundle Branch Block in Patients Undergoing Transcatheter Aortic Valve Replacement
Data from the Copenhagen City Heart Study demonstrated that RBBB was associated with an increased risk for all-cause mortality and adverse cardiovascular outcomes in the general population.70 A large meta-analysis of 19 prospective cohort studies confirmed the same findings; RBBB was associated with an increased risk of mortality in the general population and in patients with heart disease.71 RBBB is a well-recognized risk factor for PPI or late bradycardia in post-TAVR patients.24,72,73 Watanabe et al. evaluated the prognostic effect of pre-existing RBBB in patients undergoing TAVR in a substudy of the Optimized Transcatheter Valvular Intervention (OCEAN-TAVI) registry, which used the SAPIEN XT prosthesis.74 Of 749 patients, 102 (13.6%) had pre-existing RBBB, and this group had a higher incidence of PPI than the group without RBBB (17.6% versus 2.9%).74 Patients with RBBB demonstrated an increased risk of cardiovascular mortality after TAVR, and were at higher risk of cardiac death if discharged without pacemakers (HR 2.6).74 A recent study showed a similar result; RBBB was present on baseline EKG in approximately 10% of patients and associated with higher 30-day rates of PPI and death.75 Patients with pre-existing RBBB should be carefully monitored to detect fatal arrhythmic events after discharge and may require prolonged hospitalization.
Predictors and Outcomes of Permanent Pacemaker Implantation Following TAVR
Positive predictors of PPI post-TAVR are age, male sex, baseline conduction disturbances, intraprocedural atrioventricular block (AVB), narrow LVOT, the severity of mitral annular calcification, and use of self-expanding valve (Table 2).24,76,77 Patients who received PPI were more likely to have larger THVs implanted, higher oversizing, larger left ventricular internal diastolic dimensions, larger aortic valve annular size, larger aortic valve area, and lower aortic valve mean gradient.76 Moreover, septal bulge can result in a smaller LVOT and increased prosthesis:LVOT diameter ratio, which increases risk of PPI.73 PPI was associated with longer hospital and intensive care unit stays,73,76 and significantly increased cost associated with TAVR.78–80 Most studies reported a median time of 3 days from TAVR to PPI, and almost 90% of PPIs were performed within 7 days of TAVR.73,76,81,82 It is believed that conduction abnormalities occurring at a later time are a result of edema and late expansion of the THV prosthesis.83,84
Chronic right ventricular pacing causes electrical and mechanical dyssynchrony, and has been associated with a deleterious effect on left ventricular function and an increased risk of heart failure hospitalizations in patients with pre-existing heart failure.85–87 Among TAVR patients, several studies have shown a negative effect of PPI on left ventricular function at both short- and long-term follow up.30,81,88–90 A retrospective cohort study of patients undergoing TAVR at 229 sites in the US was performed using the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) registry and the Centers for Medicare and Medicaid Services database. The study found that PPI was required within 30 days of TAVR in 6.7% of cases and varied among those receiving self-expanding THVs (25.1%) versus balloon-expanding THVs (4.3%).76 Early PPI is a common complication following TAVR and was associated with higher mortality and composite endpoint of mortality or heart failure admission at 1 year.76
Conversely, another recent multicenter study showed PPI was associated with an increased risk of heart failure rehospitalization and lack of LVEF improvement, but not total mortality or cardiac mortality, after a median 4-year follow up.90 A meta-analysis by Regueiro et al. also failed to show any association between PPI and mortality (total and cardiovascular),41 which was similar to the Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) trial that did not show any effect of new PPI post-TAVR on 2-year mortality.4
A study from the PARTNER trial and registry confirmed PPI after TAVR had higher rates of repeat hospitalization and a longer duration of hospitalization, but did not show any association with 1-year mortality.73 Whether more long-term follow-up is needed to better evaluate this risk of PPI on post-TAVR mortality is yet to be determined, particularly as the therapy extends to low-risk aortic stenosis patients.
Management of Conduction Abnormalities After Transcatheter Aortic Valve Replacement
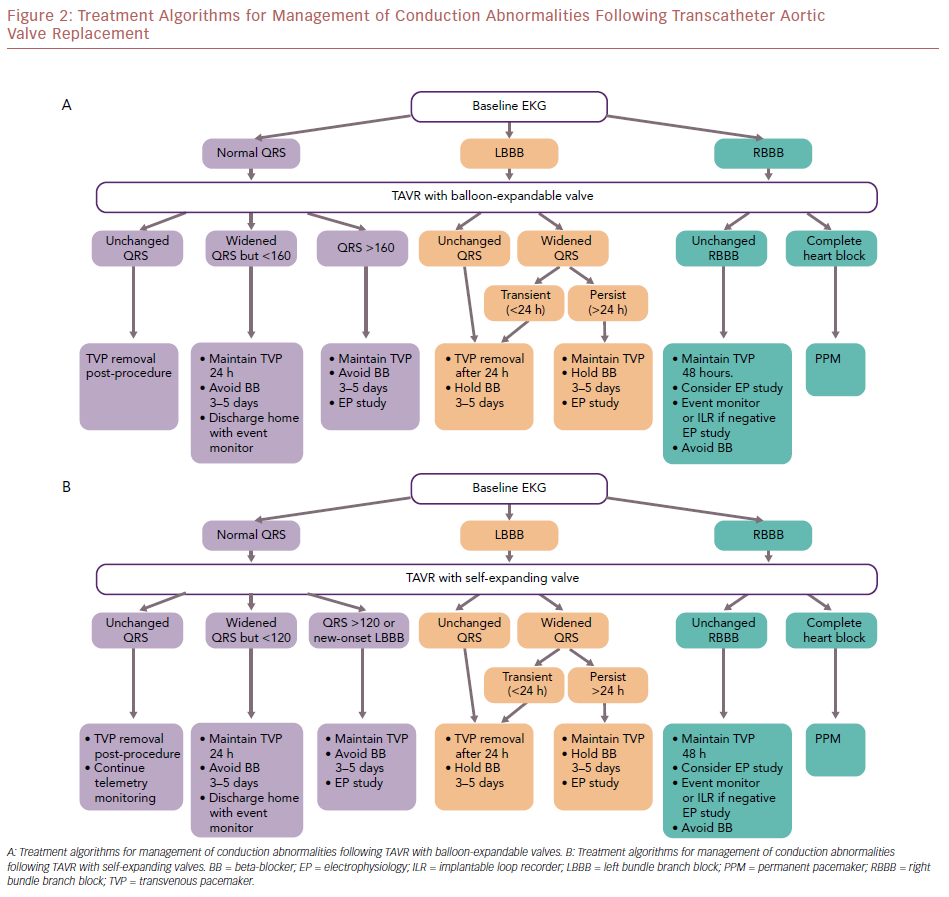
Toggweiler et al. evaluated a cohort of 1,064 patients who underwent TAVR; 6.7% of patients developed delayed HAVB, of which most cases occurred within the first 48 hours, and 2.3% had HAVB at 3–8 days post-TAVR.91 The rates of delayed HAVB in patients with complete RBBB, LBBB, and without bundle branch block (BBB) are 27%, 11%, and 2%, respectively.91 A first-degree AVB was associated with a higher probability of subsequent HAVB.91 Overall, the presence of conduction disorders (BBB, first-degree AVB, or bradycardia in patients with AF) on the EKG post-TAVR had high sensitivity (99.0%) and negative predictive value (99.7%) for the occurrence of delayed HAVB.91 The authors propose a treatment algorithm for the management of conduction abnormalities post-TAVR (Figure 2).
Without Conduction Disorders
Patients without new BBB and first-degree AVB did not develop HAVB at 30 days post-TAVR.91 Moreover, the rate of HAVB was low in patients with AF without BBB or bradycardia.91 The temporary venous pacemaker (TVP) can be removed immediately post-procedure, and telemetry monitoring and a daily 12-lead EKG can be continued. Patients without new BBB and first-degree AVB may be candidates for early discharge.
With a New Left Bundle Branch Block or First-degree Atrioventricular Block
The risk of HAVB is highest in patients with pre-existing RBBB, followed by those with LBBB, and first-degree AVB. It is recommended that TVP is maintained in patients with RBBB and LBBB, and that telemetry monitoring and a daily 12-lead EKG is continued. Avoid atrioventricular nodal blocking agents. In patients with a balloon-expandable THV, consider electrophysiology study if there is a worsening PR interval or the PR interval is >200 ms, or QRS duration is >160 ms in the first 48 hours. However, if the PR interval is stable and QRS duration is <160 ms in the first 48 hours, patients can be discharged home with an event monitor. In patients with a self-expandable THV, consider electrophysiology study with development of a new LBBB. A HV interval >65 ms may be suggestive of a significant conduction abnormality and warrant PPI; patients with a HV interval <65 ms can be discharged home with an event monitor. High-risk patients, such as those with baseline RBBB and bifasicular blocks, may warrant long-term monitoring with an implantable loop recorder if early event monitoring is unremarkable.
With Post-procedural High-grade Atrioventricular Block
PPI is indicated for either third-degree or advanced second-degree AVB at any anatomic level, which is not expected to resolve, or in the presence of sinus node dysfunction and documented symptomatic bradycardia.92
TAVR represents a valid option for treatment of severe symptomatic aortic stenosis. Post-TAVR conduction abnormalities are still a common complication following both self- and balloon-expandable THVs. Predictors of TAVR-induced LBBB and PPI depend on baseline patient characteristics such as preoperative EKG pattern, anatomy of the AVN, His bundle, and surrounding structures, as well as intra-procedural technical factors.
There is no consensus on how to prevent and/or treat post-TAVR conduction abnormalities. Protocols vary among operators and valve centers. New generation THVs and modified techniques for valve implantation may help to reduce the prevalence of PPI. Further studies are required to validate and establish universal algorithms to manage conduction abnormalities following TAVR, irrespective of the prosthesis type.