The incidence of congenital heart disease (CHD) is slightly less than 1% of all live births.1 With extensive medical and surgical innovation over the past 50 years, and the high-quality care received in childhood, 85–90% of children with CHD in the US survive into adulthood. As a result, the number of adults with congenital heart defects in the US now exceeds 1 million, with a projected continued growth rate of 5% per year.1,2 Coarctation of the aorta (CoA), considered a moderately complex form of CHD, was first described by Morgagni in 1760.3 It occurs in approximately 0.04% of the population, and accounts for approximately 5% of all congenital heart defects.4 It makes up approximately 10% of lesions in adults with CHD (ACHD), and occurs twofold to fivefold more often in men than in women.5
CoA can occur as an isolated lesion or as a part of a complex defect in the presence of other intracardiac lesions. Most commonly, this includes bicuspid aortic valve (≤85%), mitral valve abnormalities, or ventricular septal defects. Others include transposition of the great arteries, Taussig–Bing anomaly, and hypoplastic left heart syndrome. Patients with Turner’s, Noonan’s, and Williams–Beuren syndromes can have CoA, and various gene deletions have been described. One such gene mutation involves the NOTCH1 gene.6-10
Morphology
The morphology of CoA is a spectrum, and varies widely based on the age at presentation. For example, transverse arch and isthmus hypoplasia is most commonly seen in the newborn period, whereas discrete localized stenosis or membrane-like obstruction just beyond the left subclavian artery is more often encountered in older children and adults.11,12 More unusual locations include the ascending aorta and abdominal aorta. The etiology of CoA remains unclear. The primary mechanism is thought to be medial thickening and intimal hyperplasia that leads to the formation of a posterolateral ridge that encircles the aortic lumen, resulting in the CoA segment. Another theory is that of aberrant ductal tissue and abnormal intrauterine blood flow through the aortic arch.8,9
Presentation
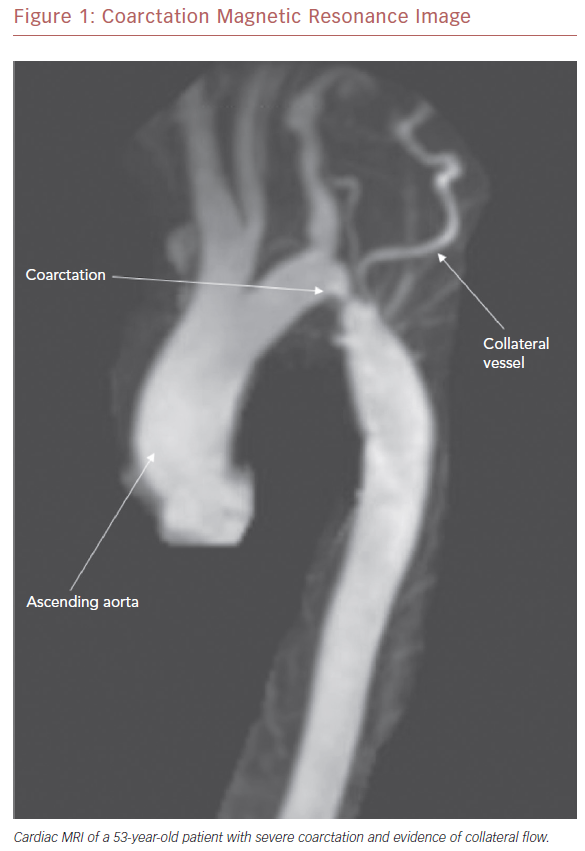
Although most cases of CoA are diagnosed in infancy, some patients may not present until later childhood, adolescence, or adulthood (Figure 1). The age at presentation is primarily dependent upon the degree of stenosis across the coarcted segment. For example, severe CoA can lead to heart failure and shock upon closure of the ductus arteriosus in infancy.13 Milder forms, in contrast, may not be detected until the patient is much older.
Patients with extensive collateral vessels that bypass the area of CoA can remain asymptomatic for many years.14,15 Indeed, although the natural history of untreated CoA is associated with a mean life expectancy of approximately 35 years, there are reports of untreated patients living to 92 years.16 The most common presenting finding is systemic hypertension measured in the upper extremities. Others can include cardiac failure or endocarditis.10,11,17 Older patients may complain of symptoms, such as headache, dizziness, tinnitus, abdominal angina, and exertional leg fatigue.
Management
Several treatment options for CoA are now available, and include both surgical and transcatheter interventions. The decision on the optimal treatment method can be complicated, and there is no evidence-based standard of care. In general, management is guided by the age at presentation, and the complexity of the coarctation and/or associated defects.
Surgical Intervention
The first surgical repair of CoA was performed in 1944 by Crafoord and Nylin.18 Over the ensuing 40 years, refinement in surgical techniques expanded the available surgical options and included patch augmentation, end-to-end anastomosis, and even the insertion of a Gore-Tex bypass graft with more complex anatomy.12 Another surgical approach was the subclavian flap, whereby augmentation of the coarcted segment was performed using the subclavian artery as a surgical flap.19 Today, the surgical risk is estimated to be <1% in patients with simple CoA, and surgery remains the preferred method of treatment in infants. In the critically ill infant, older child, or adolescent, however, a less invasive method employing transcatheter treatment is often favored.20–22 This is particularly true after the third or fourth decade of life, when intraoperative morbidity and mortality increases as a result of degenerative aortic wall changes and comorbid conditions, such as systemic hypertension, heart failure, and coronary artery disease. Complications from coarctation surgery can include spinal cord damage, pleural effusions, phrenic, or laryngeal nerve paralysis, paradoxical hypertension, and infection.23 As a result, such older patients are often best managed by non-surgical approaches.
Transcatheter Treatment
Transcatheter treatment of CoA became popular with the advent of balloon angioplasty by Lock et al. in 1983.24 In fact, for patients aged >4 years or weighing >15 kg, transcatheter therapy for both native and especially recurrent CoA has become the standard of care in the majority of institutions.25 Guidelines published in 2011 by the American College of Cardiology/American Heart Association for transcatheter treatment for primary and recurrent CoA further allowed for balloon angioplasty and/or stenting when a peak-to-peak gradient of ≥20 mmHg at the time of cardiac catheterization was evident. It should be noted, however, that interventions may be warranted with less severe gradients if there is systemic hypertension with imaging evidence of a significant narrowing.20
Balloon Angioplasty
Successful relief of stenosis by balloon angioplasty is accomplished by damage to the aortic intima and media, the degree of which is often difficult to control. The major drawbacks are recoil of the vessel with recurrence of stenosis and vascular injury with consequent vessel dissection or aneurysm formation.26 In addition, elastic fiber fragmentation, underlying fibrosis, cystic medial necrosis, and vascular calcification may predispose patients to dissection, aneurysm formation, or even rupture at the time of repair.12,27
Bare Metal Stent Placement
Intravascular bare metal stent placement was first performed by O’Laughlin et al. in 1993 and, at many centers, became the treatment of choice for children and adults with native CoA.28 The goal of using bare metal stents was to avoid dissection, excessive dilation, and elastic recoil of the aorta. It was also believed to result in less vascular injury than balloon angioplasty alone.29
In a study comparing surgical repair, balloon angioplasty, and stent treatment of native CoA in children, stented patients had significantly lower acute complications compared with those who had undergone surgical or balloon angioplasty repair.30 Bare metal stents were also shown to be more effective at achieving acute and sustained reductions in blood pressure gradients. As a result, stent therapy for CoA became the preferred intervention over balloon angioplasty for older children and adults.31 Nevertheless, although rare, complications such as aneurysm formation, stent malposition, vascular complications, death, and the need for emergency surgery can occur.32,33
Covered Stent Placement
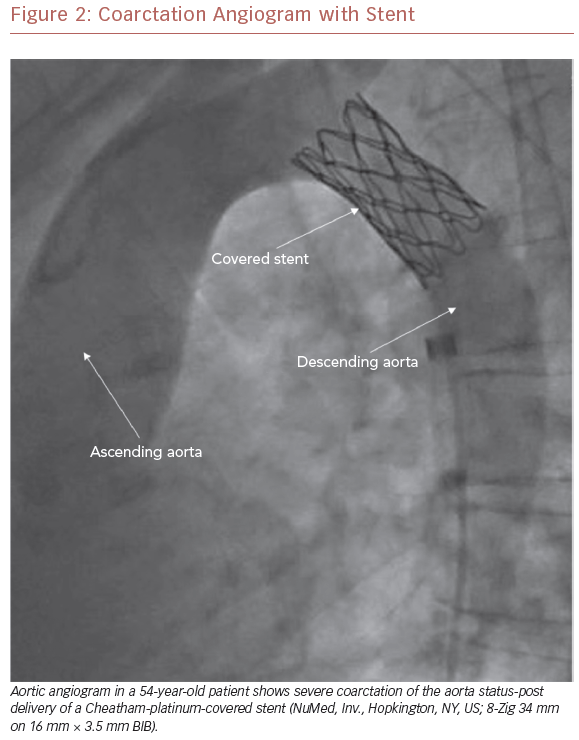
To this end, perhaps one of the most important advances in the management of CoA has been the development of covered stent technology (Figure 2). First used in 1999 to treat coexistent CoA and aneurysm of the aorta in a young man, it has since been used in a variety of settings, and an array of covered stents have become available for clinical use.34,35 Indications for the use of covered stents are now wide-ranging and continue to expand. In CoA patients, they are preferentially deployed in a number of situations, including the treatment of associated aneurysms, native CoA with near-interruption, associated patent ductus arteriosus, and in older patients due to the potential for aortic disruption (Figure 1).36,37 In addition, where exclusion of the left subclavian artery is anticipated, wire perforation of the covered stent followed by balloon angioplasty through a stent cell has allowed for a recannulized vessel.38
Covered stents can also be used as a rescue treatment in patients with stent complications and in patients with complex CoA anatomy. Potential physiological benefits following stenting include mid-term left ventricular mass regression, ventricular function improvement, and improvement in central aortic function with reduction of daytime ambulatory systolic blood pressure such that anti-hypertensive medication can often be discontinued.39,40 Follow-up with CT examination suggests that the overall development of aneurysms continues to be low.36,41–44 Despite all the potential advantages seen with covered stents, aggressive surveillance remains necessary to screen for long-term complications.
Long-term Complications
While symptoms and survival are improved after surgical or transcatheter intervention, the long-term survival of CoA patients continues to be lower than in the general population.10 Late complications can include systemic hypertension, recurrent CoA, aneurysm formation at the site of previous repair, early coronary artery disease, and intracranial aneurysm.
Systemic hypertension, even after excellent repair, remains common, and can lead to stroke, myocardial infarction, and heart failure. The estimated prevalence ranges from 25% to 68%, and is especially common in patients with concomitant aortic arch hypoplasia or in patients who underwent repair at a later age.45 It is believed that systemic hypertension results from increased aortic stiffness, decreased baroreceptor sensitivity, raised plasma concentrations of epinephrine and norepinephrine, or coexisting essential hypertension.46,47 Another factor may be a hypoplastic aortic arch that was not corrected at the time of initial treatment. As hypertension may not be evident at rest or during the routine clinic visit, ambulatory blood pressure monitoring and exercise testing to evaluate for exercise-induced hypertension are often employed.48
Even in the absence of early complications with good initial relief of obstruction, recurrent CoA and aneurysm formation at the site of coarctation repair are seen. Recoarctation at the site of previous repair or residual coarctation are important causes of morbidity, as it can lead to worsening systemic hypertension, coronary artery disease, or systolic and diastolic heart failure. Studies have found the rate of recoarctation to be between 3% and 15%.16,49–51
While recoarctation or residual stenosis may occur with all surgical techniques, a study by Choudary et al. demonstrated a lower rate of significant recoarctation with the end-to-end anastomosis repair.52 Indeed, the extended end-to-end anastomosis is typically preferred, as it allows resection of the coarctation while avoiding prosthetic material and is less prone to restenosis.20
Aneurysm and pseudoaneurysm formation, both of the ascending aorta and in the region of the aortic isthmus, if left untreated, can lead to rupture with rates up to 100% within 15 years.19 The incidence of early and late aneurysm formation after balloon angioplasty has been reported to be between 5% and 11.5%.29,53 It is felt that associated bicuspid aortic valve, aortic wall changes, and systemic hypertension are largely responsible for aneurysm formation in the ascending aorta.54
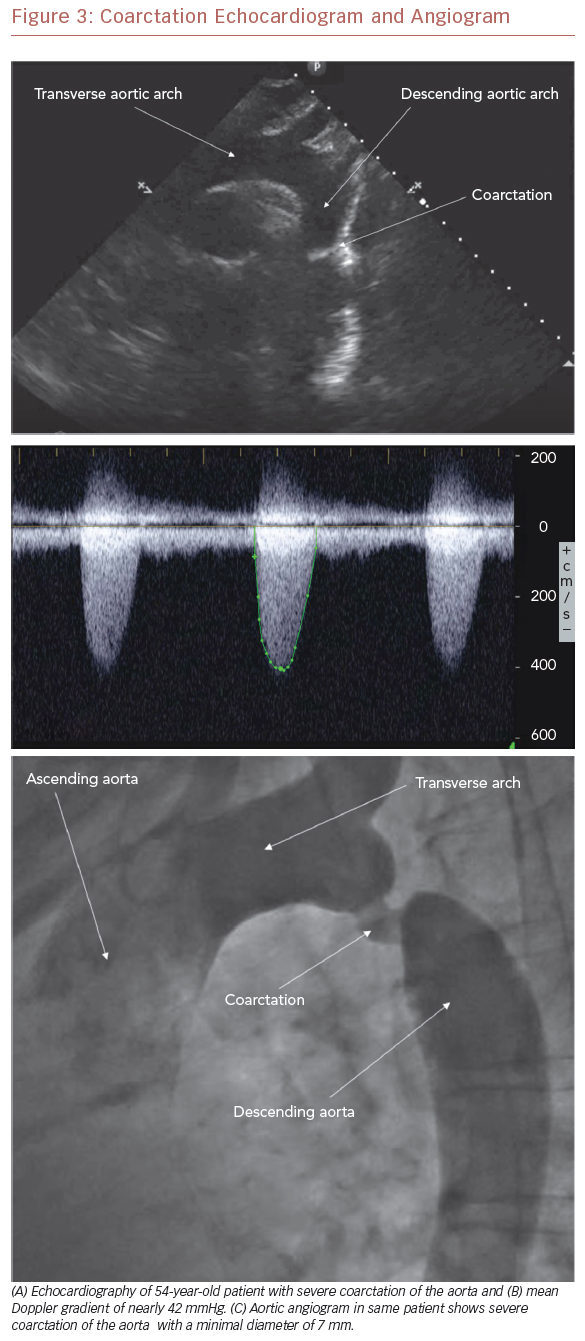
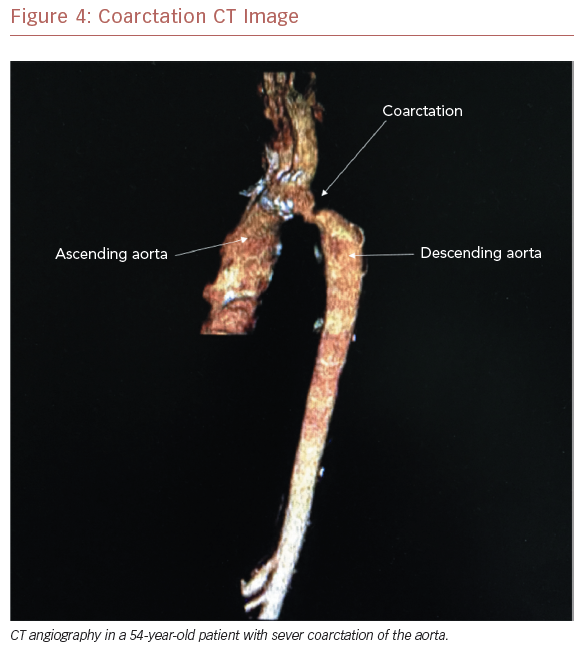
Aneurysm formation at the region of the aortic isthmus does not appear to be related to the presence of an intimal tear at the time of the initial procedure and, indeed, the majority of aneurysms tend to be small and discrete. Still, intervention is required for late aneurysm formation in patients after balloon dilation of native recurrent CoA.55 As with recoarctation, most surgical types carry a risk of aneurysm formation. It is felt, however, that the end-to-end anastomosis repair may carry the lowest risk.52 Because of the risk of recoarctation, residual stenosis, and aneurysm formation, all patients need careful follow-up after coarctation repair. While evidence of recoarctation may be detected by a clinical examination and echocardiography (Figure 3A–C), cardiac MRI (CMR) (Figure 1) or CT angiography (CTA) (Figure 4) are recommended to evaluate for aneurysm and/or pseudoaneurysm formation.56
Early coronary artery disease (CAD) is also described.12,57,58 The etiology of CAD is not well understood, but thought to be related to long-standing hypertension. In a study from the Mayo Clinic, CAD was the most common cause of late postoperative death.59 A Canadian study later found that CoA was not a sole predictor for the development of coronary artery disease, and called into question the prevalence of CAD in patients with a history of CoA.60
Finally, there is an association of CoA and extracardiac lesions, mainly intracranial aneurysms (i.e. berry aneurysm of the circle of Willis) in up to 10% of patients.10 The reason for this is not clear, but might be a consequence of e has been found to be a risk factor.61 Aneurysm size tends to increase with age, and uncontrolled hypertension increases the risk of rupture.62 Given this, screening for cerebrovascular aneurysms is recommended.56 It should be noted, however, that routine screening for aneurysms remains a matter of debate amongst some practitioners.63
Follow-up Assessment
Whether the patient has undergone a surgical or transcatheter treatment, follow-up care is required such that long-term complications can be identified and treated. The type and frequency of testing is largely guided by symptoms and the clinical examination, although often includes additional testing to evaluate for issues that may not be evident by physical findings or echocardiography alone. Resting blood pressure, measured in the upper and lower extremities, is important, as a systolic blood pressure discrepancy along with a delay between the brachial to femoral pulses and/or vascular murmur heard between the scapulae could hint toward recoarctation or residual stenosis.13,64 As hypertension may not be evident at rest or during the routine clinic visit, ambulatory blood pressure monitoring and exercise testing to evaluate for exercise-induced hypertension are often employed.48
Electrocardiography may demonstrate left atrial or ventricular pressure load or left ventricular strain. Chest radiography, while non-specific in the young patient, may demonstrate the ‘figure of 3’ sign beneath the aortic knob, which is characteristic of native or recoarctation. Rib notching due to dilated intercostal arteries may also be present in older patients.22,65 Given the widespread availability and ease of use, the mainstay of the imaging evaluation remains the echocardiogram (Figure 3A and 3B). From the suprasternal notch view, the anatomy of the site of CoA is often well visualized, and a mean Doppler gradient >20 mmHg or mean Doppler gradient >10 mmHg in the setting of left ventricular systolic dysfunction or aortic valve regurgitation suggests that intervention may be necessary.48,66,67 To further delineate the anatomy, the echocardiographic evaluation should be coupled with advanced imaging, such as CMR or CTA (Figure 4). Indeed, CMR and CTA have become the preferred non-invasive means to evaluate coarctation before and after operative and transcatheter treatment in adults. This is particularly important to evaluate for the presence of aneurysm and/or pseudoaneurysm formation. At cardiac catheterization, a significant coarctation is defined as a peak-to-peak gradient >20 mmHg across the coarctation in the absence of significant collateral circulation. While this type of diagnostic testing was frequently used in the past, it is now primarily reserved for intervention or where additional hemodynamic information is required.55,65
Conclusion
CoA is a complex lifelong heterogeneous disease process with multiple associated complications both with and without treatment. Although significant advances in the recognition and treatment of patients with CoA have taken place, continued and aggressive screening and management of patients with CoA are vital. Follow-up needs to include thorough evaluation for late complications, including systemic hypertension, coronary artery disease, recurrent obstruction, and aneurysm formation. Given the limitation of clinical examination and echocardiography alone, advanced imaging techniques, including CMR or CTA, are necessary. A thorough evaluation is particularly important whenever systemic hypertension is present to ensure that recoarctation or residual stenosis is not present. Treatment, if necessary, should be performed in centers with extensive experience with CoA.