Ventricular tachycardia (VT) occurs most frequently in patients with structural heart disease. Management of VT in these patients can be complex, requiring an integrated approach with multiple therapeutic modalities. Antiarrhythmic drugs (AADs) can be effective in the management of VT and implantable cardioverter defibrillators (ICDs) have been shown to effectively prevent sudden cardiac death due to ventricular arrhythmias.1–3 Unfortunately, ICDs do not prevent the recurrence of episodes of VT and appropriate ICD shocks are associated with significant morbidity and increased rates of mortality.4,5 AADs can be used to minimize the frequency of ICD shocks, but long-term use may be required to achieve continued VT suppression and these medications can have substantial side effects.
Radiofrequency catheter ablation of VT is an effective treatment method for patients with VT in the setting of structural heart disease.6,7 Although there is limited evidence that catheter ablation improves overall mortality,8 catheter ablation is clearly effective in reducing VT burden and decreasing the number of appropriate ICD therapies. As technology and procedural techniques have improved over time, catheter ablation for VT has become an increasingly utilized treatment strategy. While optimal timing of VT ablation remains debated, it is often considered only late in the course of progressive structural heart disease, especially at institutions with less experience in the procedure.9,10 In this review, we discuss the management of VT with a focus on patients with underlying structural heart disease, including the use of AADs and ICDs. We also review the basics of VT ablation, the evidence behind the procedure, and future directions in the field.
Initial Management of Ventricular Tachycardia
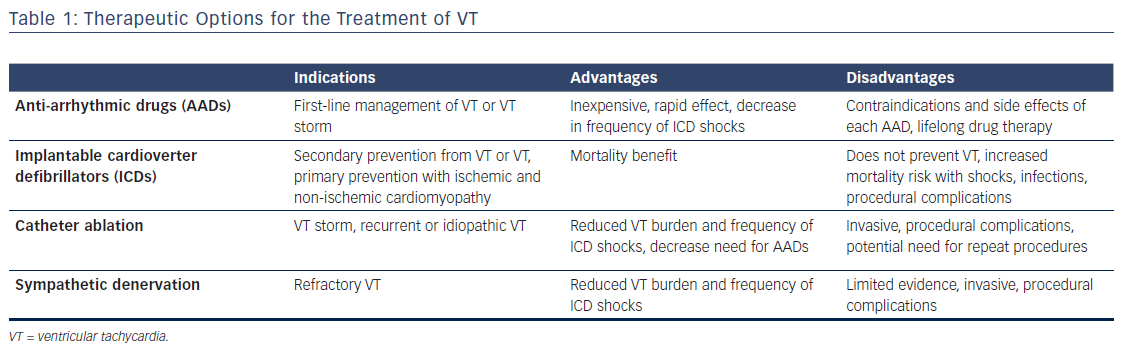
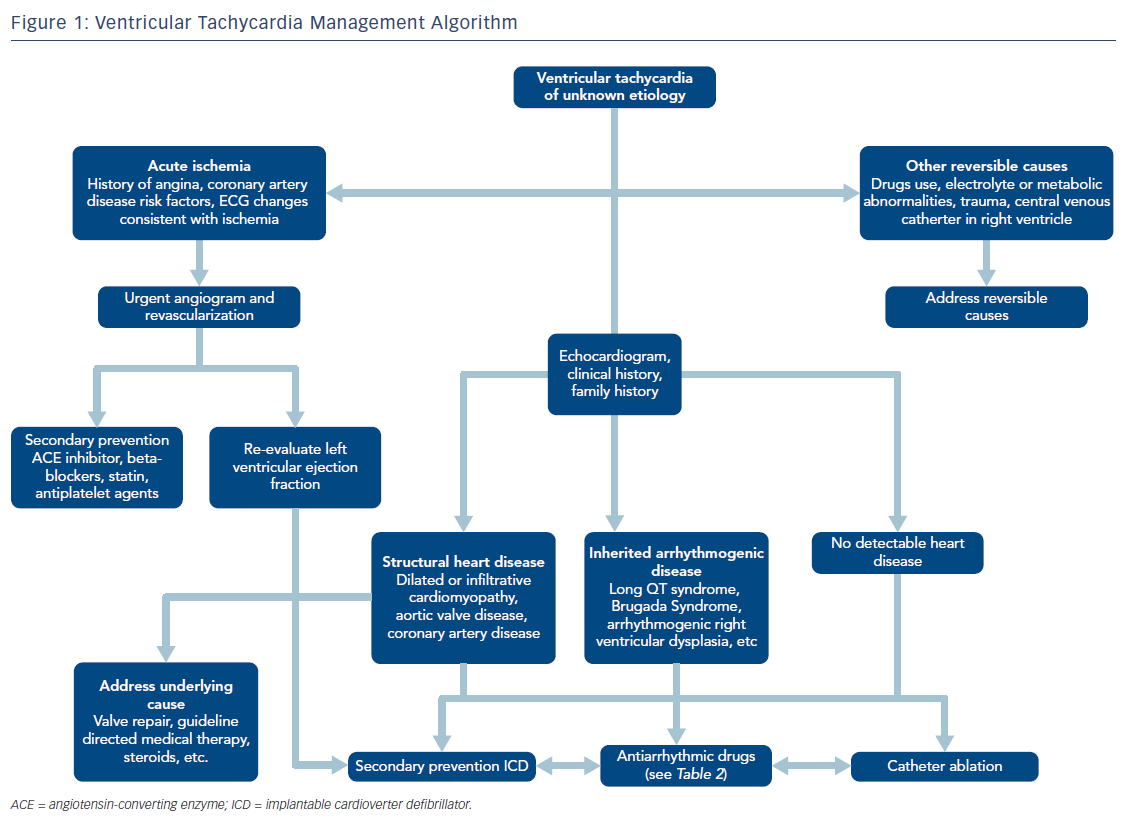
Once a diagnosis of VT is made, acute management is initially focused on achieving hemodynamic stability. If the VT causes hemodynamic instability – a function of both characteristics of the arrhythmia (especially rate) and the patient’s underlying cardiac function – electrical cardioversion can successfully restore sinus rhythm, at least temporarily. Patients who are hemodynamically unstable during VT or those with major comorbidities should be admitted to an intensive care unit where metabolic, respiratory, or other circulatory derangements should be immediately identified and corrected. Further acute management generally focuses on AADs, reprogramming of ICDs to decrease the frequency of recurrent shocks, and termination of clinically significant arrhythmias with anti-tachycardia pacing, and occasionally catheter ablation (Table 1 and Figure 1).11,12
The use of ICDs has been shown to substantially improve mortality and decrease the risk of sudden cardiac death in patients with VT both for primary and secondary prevention.1,2,5 Even with ICDs in place, AADs are also generally required both in the acute and long-term management of VT to decrease the rate of recurrent arrhythmias. AADs can reduce the incidence of both appropriate and inappropriate ICD shocks. However, all AADs may cause side effects and proarrhythmic effects have been reported in nearly 10 % of patients treated with AADs for ventricular arrhythmias.13,14
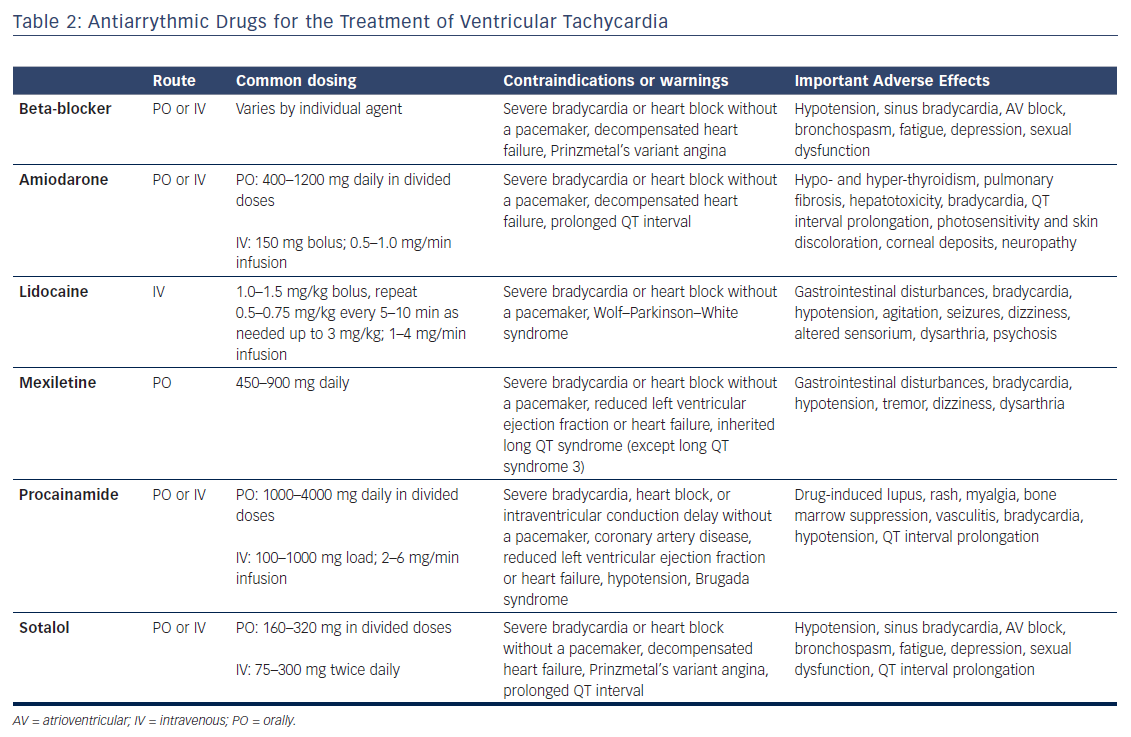
Beta-blockers effectively suppress adrenergic tone, which can be markedly increased acutely in the setting of VT. Beta-blockers also have long-term benefits, especially in patients with underlying structural heart disease and reduced left ventricular systolic function.15,16 Amiodarone is perhaps the most widely used AAD for VT and can be safely used in the acute setting with impressive efficacy.17,18 Several issues arise with amiodarone use. Acute use of amiodarone can cause hypotension and hemodynamic instability, and, in some patients, can also increase defibrillation thresholds. Serious long-term adverse effects of the medication include liver dysfunction, thyroid disease, pulmonary fibrosis, and ophthalmologic complications.19,20 Lidocaine is an intravenously delivered class 1B AAD that can be effective especially for the treatment of ischemic VT. Importantly, central nervous system side effects of hallucinations, tremors, or seizures can occur with high doses.21,22 For this reason, monitoring lidocaine levels in the blood is recommended. Mexiletine is a similar class IB agent that can be administered orally. While the efficacy of mexiletine when given as monotherapy is limited, it can result in improved VT suppression when used in conjunction with amiodarone. Procainamide, a class IC agent that has also been shown to be effective for the acute management of VT, although acute use can be limited by hypotension and chronic use by a number of side effects including drug-induced lupus, gastrointestinal disturbances, and hematologic abnormalities (Table 2).23,24
Catheter Ablation for Ventricular Tachycardia
Although some form of AAD combined with ICD therapy is currently the standard of care for the management of VT, it is important to remember that both of these treatments are not true cures for the disorder. Continued AAD use may be necessary to achieve long-term VT suppression, but long-term side effects must be considered, especially as many patients with VT also have significant pulmonary, renal, or hepatic dysfunction. Catheter ablation is an invasive strategy that offers the potential of cure by destroying or isolating arrhythmogenic tissue. Successful elimination of VT with catheter ablation can allow for reduction or discontinuation of AADs, thus removing the risks of developing side effects with long-term AAD use.25 In recent years there has been a growing role for catheter-based ablation both in the acute and long-term management of VT.9 This growth is due in part to marked improvement in the knowledge of the pathological basis of arrhythmias, as well as the continued evolution in our ability to identify the origin of an arrhythmia and destroy or isolate tissue necessary for arrhythmia generation or propagation.26
Catheter ablation can be especially useful in the management of VT storm in patients with ischemic and non-ischemic cardiomyopathy.27,28 VT storm is defined as at least three distinct episodes of sustained VT or ventricular fibrillation within the last 24 hours or the occurrence of incessant VT for at least 12 hours.29 Initial management focuses on achieving hemodynamic stability, which may include intra-aortic balloon pumps (IABPs), percutaneous left ventricular assist devices (pLVADs), or extracorporeal membrane oxygenation (ECMO).12 AADs and ICD reprograming are also essential, although catheter ablation has a growing role given the superiority over medical therapy in reducing arrhythmic burden.6,8
In order to maximize the likelihood of successful catheter ablation, precise location of the suspected arrhythmogenic foci or circuits is necessary. This is achieved through a variety of mapping techniques including activation mapping, pace mapping, entrainment mapping, and substrate mapping. In activation mapping, VT is induced with ongoing electrogram analysis to determine the VT circuit or site of earliest activation representing the VT exit site. Pace mapping involves pacing at different sites in the ventricles to identify the site with the best match, representing the site of origin of a focal arrhythmia or the exit site of a reentrant arrhythmia.30,31 Entrainment mapping involves identifying critical sites of reentrant VT circuits by examining responses to ventricular overdrive pacing during sustained VT.32,33 Finally, substrate mapping is commonly used when VT cannot be induced or sustained VT cannot be hemodynamically tolerated. In these cases, arrhythmogenic substrate is indirectly identified by the presence of abnormal electrograms (e.g. fractionated, low-voltage potentials, or late signals after the QRS), or evidence of scar on imaging.34–36 Importantly, VT must be inducible, sustained, and hemodynamically stable in order for activation and entrainment mapping to be performed. In cases in which VT is not tolerated due to hemodynamic compromise, hemodynamic-support devices such as ECMO, IABP, or pLVAD (i.e. Impella or Tandemheart) can be helpful. Pre-procedural magnetic resonance imaging can also be used to guide ablation. Late-gadolinium enhancement in both ischemic and non-ischemic cardiomyopathy can accurately localize potentially arrhythmogenic substrate, and its use has been associated with improved ablation outcomes.37–39
The most commonly used energy for catheter ablation at the current time is radiofrequency energy, which utilizes alternating current to deliver energy at the catheter tip with subsequent thermal injury and coagulation necrosis. Importantly, this method is critically dependent on contact with the target myocardium and stability during ablation.40,41 Other forms of ablation including DC ablation (electrical energy) or cryoablation (freezing to achieve necrosis) have also been used in certain settings.40,42,43 Other strategies including electroporation and noninvasive external radiotherapy ablation have also been shown to be effective in preliminary studies and may be options to consider in the near future.
Catheter ablation procedures are associated with several notable complications that must be considered, although the rates of these complications are low. Vascular injury at the access site is the most common complication, due to the fact that multiple large-diameter femoral venous and arterial sheaths may be necessary and intraprocedural anticoagulation is often required. The Multicenter European Radiofrequency Survey (MERFS) examined complication rates for all types of radiofrequency catheter ablations and found that death occurred in 0.13 %, embolic stroke in 0.5 %, cardiac tamponade requiring pericardial drainage in 0.8 %, complete heart block in 0.6 %, and arterial or venous thrombosis in 0.4 %.44 Importantly, rates of complication are heavily influenced by patient and arrhythmia characteristics as well as degree of operator and center experience. While older patients are likely to have more comorbidities, our experience has shown that VT ablation can be safely and effectively performed, thus older age alone should not exclude patients from being offered VT ablation.45
Evidence for Catheter Ablation
Catheter ablation for VT has been performed with increasing frequency over the past few years. Most evidence to date demonstrates the benefit of ablation with reduction in recurrent VT and thus appropriate ICD shocks; however, improvement in overall survival, quality of life, or healthcare costs has not been definitively shown (Table 3). Substantial heterogeneity of patient and arrhythmia characteristics as well as ablation techniques utilized limit the interpretability of many of the observational studies that have been published; however, available data suggest improved freedom from VT and transplant-free survival.6,46 Standards have been proposed for trials to report specific clinical characteristics (e.g. type and severity of VT), procedural techniques (e.g. mapping criteria and definition of procedural success), and efficacy endpoints (e.g. mortality and specifics of VT recurrence).47
To date, there have been four major prospective randomized controlled trials evaluating catheter ablation for VT in patients with ischemic cardiomyopathy. In the first, the Substrate Mapping and Ablation in Sinus Rhythm to Halt Ventricular Tachycardia (SMASH-VT) study, relatively low-risk patients with prior MI who had a history of a single episode of a ventricular arrhythmia or appropriate ICD therapy, but had not received therapy with AADs (class I or III) were randomized to either substrate-based endocardial ablation or standard medical therapy.48 Although there was a significant decrease in the likelihood of recurrent VT requiring ICD therapy (67 versus 88 %; p=0.007), the trial was not powered for mortality, and the exclusion of patients receiving AADs severely limits the clinical relevance of this trial.
The Ventricular Tachycardia Ablation in Coronary Heart Disease (VTACH) study prospectively evaluated patients with a prior MI, reduced left ventricular ejection fraction (<50 %), and stable VT who would otherwise qualify for a secondary prevention ICD.49 All patients were eligible to receive AADs and the ablation technique was at the discretion of the operator. Of the 107 patients included in the analysis with approximately 2 years of follow-up, the 52 in the ablation group had improved freedom from recurrent ventricular arrhythmias (47 versus 29 %; p=0.04), but there was no significant difference in mortality rates. Although the VTACH study demonstrated the ability for catheter ablation to substantially reduce the risk of recurrent VT, it is equally important to note the ≥50 % risk of VT recurrence within 2 years and associated need for ICDs in these patients regardless of whether ablation was performed.
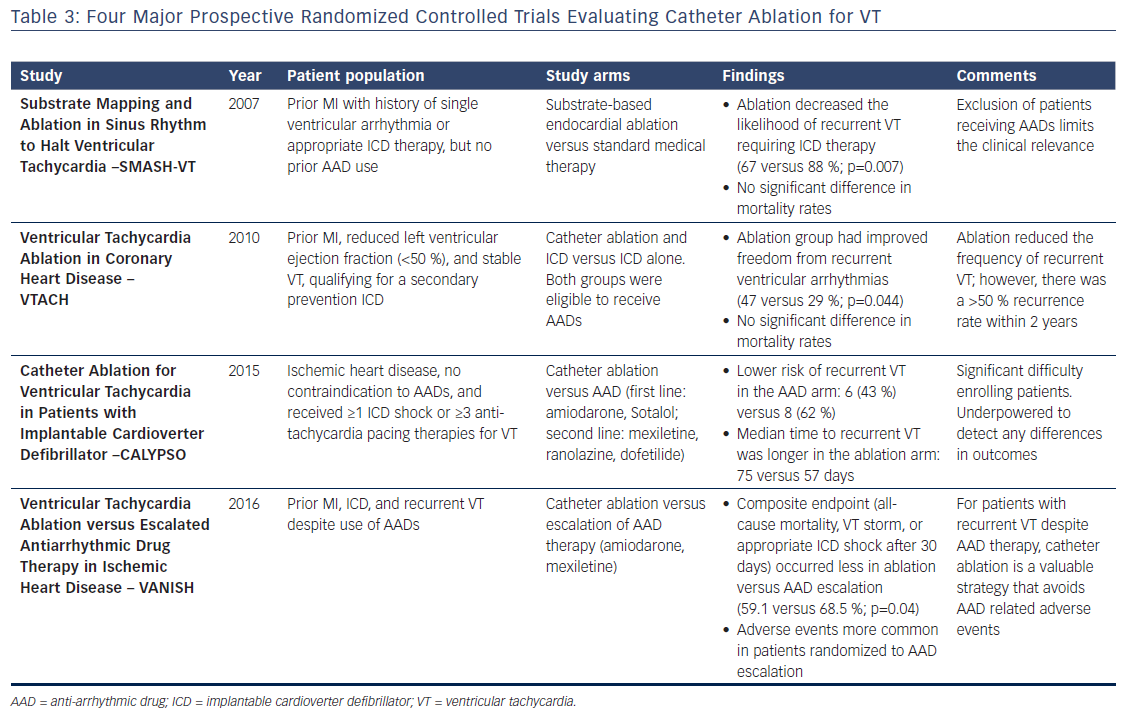
The Catheter Ablation for Ventricular Tachycardia in Patients with Implantable Cardioverter Defibrillator (CALYPSO) trial was designed to determine the feasibility of conducting a prospective trial comparing early ablation versus AADs in patients with ischemic cardiomyopathy, no contraindication to AADs, and ICDs that had delivered appropriate therapy.10 The study was terminated prematurely after having experienced challenges with enrollment – over nearly 2 years, 243 patients were screened, 27 enrolled, and only 17 completed 6 months of follow-up. Although underpowered to detect any differences in outcomes, CALYPSO highlighted an important clinical limitation for enrollment in trials evaluating early VT ablation – this procedure is rarely considered in patients who have not already failed AAD therapy.
The Ventricular Tachycardia Ablation Versus Escalated Antiarrhythmic Drug Therapy in Ischemic Heart Disease (VANISH) trial asked a clinically relevant question about patients with a history of ischemic cardiomyopathy and recurrent VT despite use of AADs.50 Patients were randomized to catheter ablation versus escalation of AAD therapy, which included the initiation of amiodarone in patients not already taking amiodarone, or the addition of mexiletine in patients already taking amiodarone. Among 259 patients over approximately 28 months of follow-up, the primary outcome, a composite endpoint of all-cause mortality, VT storm, or appropriate ICD shock after 30-day treatment period, occurred in fewer patients who were randomized to ablation versus AAD therapy escalation (59.1 versus 68.5 %; p=0.04). Importantly, while ablation-related procedural complications (e.g. bleeding, vascular injury, or cardiac perforation) did occur, treatment-related adverse events were more common in patients randomized to AAD therapy escalation. Importantly, the VANISH trial demonstrated that among high-risk patients with recurrent VT despite AAD therapy catheter ablation is a valuable strategy that avoids some of the adverse events associated with AAD therapy.
There are many obstacles to completion of randomized controlled trials for VT ablation. In fact, a recent systematic review of clinical trials for VT ablation identified 15 randomized controlled trials comparing ablation in the National Institutes of Health or International Standard Randomised Controlled Trial Number registry.6 Only the four studies discussed above have been completed, three are ongoing, and the remaining eight have either been terminated or have an unknown status. To increase the number of VT ablation studies and improve the evidence for catheter ablation, changes are necessary in enrollment strategies, provider education, and standardized reporting outcomes, as mentioned earlier.
Future Directions
Many unanswered questions remain regarding the efficacy and safety of VT ablation due to inadequate evidence randomized controlled trials to guide management. The majority of studies to date, including all the major randomized controlled trials, have only enrolled patients with ischemic cardiomyopathy. Randomized controlled trials are needed in patients with non-ischemic cardiomyopathies as management varies significantly in current clinical practice; although, our group has shown substantial reduction in VT burden following catheter ablation in these patients.51 The ideal timing of ablation in the natural history of VT and patient characteristic, including age, and comorbidities need to be examined in greater detail. Patients are often referred for VT ablation late in their clinical course, and some studies have suggested improved outcomes with earlier referral after onset of VT.52,53 Additionally, long-term outcomes for patients should be explored with extended follow-up included in trials evaluating not only mortality and recurrent VT, but also the need for repeat ablation and quality of life after ablation.
The role of alternative treatment strategies for refractory VT such as sympathetic denervation also warrants additional investigation. Sympathetic activity is critically involved in both the initiation and maintenance of VT and decreased sympathetic tone, through epidural anesthesia or cardiac sympathetic denervation, has been shown to reduce VT burden and frequency of ICD shocks.54–57 Sympathetic denervation involves removal of the lower third of the stellate ganglion and T2–T4 thoracic ganglion most often through a video-assisted thorascopic approach. Further investigation is needed to determine the ideal approach, laterality of denervation, and patient population that would benefit most from this procedure.57,58
Conclusion
Catheter ablation for VT is a valuable technique that has been clearly shown to decrease the frequency of VT recurrence and ICD shocks. Its use will continue to expand as techniques and operator experience improve over time. It is important for cardiologists to be aware of catheter ablation as a therapeutic option for VT and to refer early in the course of the disease to centers that have experience with the procedure so that all management options can be considered. Further research is essential to address the ideal timing, technique, efficacy, and safety of this procedure.